RAINBOW trial: Efficacy
CYRAMZA as a single agent, or in combination with paclitaxel, is indicated for the treatment of patients with advanced or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma with disease progression on or after prior fluoropyrimidine- or platinum-containing chemotherapy.
RAINBOW Trial Design (N=665)1,5
The phase III RAINBOW trial evaluated the efficacy and safety of CYRAMZA + paclitaxel vs placebo + paclitaxel in patients with locally advanced or metastatic gastric or GEJ adenocarcinoma with disease progression on or after prior fluoropyrimidine- and platinum-containing chemotherapy. Major efficacy outcome measure was OS. Supportive efficacy outcome measures were PFS and ORR. All patients were ECOG PS 0 or 1. Prior to enrollment, 97% of patients had progressed during treatment or within 4 months after the last dose of first-line chemotherapy for metastatic disease. Twenty-five percent of patients had received anthracycline in combination with platinum/fluoropyrimidine therapy, while 75% did not. Patients were randomized 1:1 to CYRAMZA 8 mg/kg (n=330) or placebo (n=335) every 2 weeks (on days 1 and 15) of each 28-day cycle. Patients in both arms received paclitaxel 80 mg/m2 on days 1, 8, and 15 of each 28-day cycle.1,5
Adding CYRAMZA to paclitaxel increased median OS by 30%1
versus placebo + paclitaxel - that could mean more time for your patients
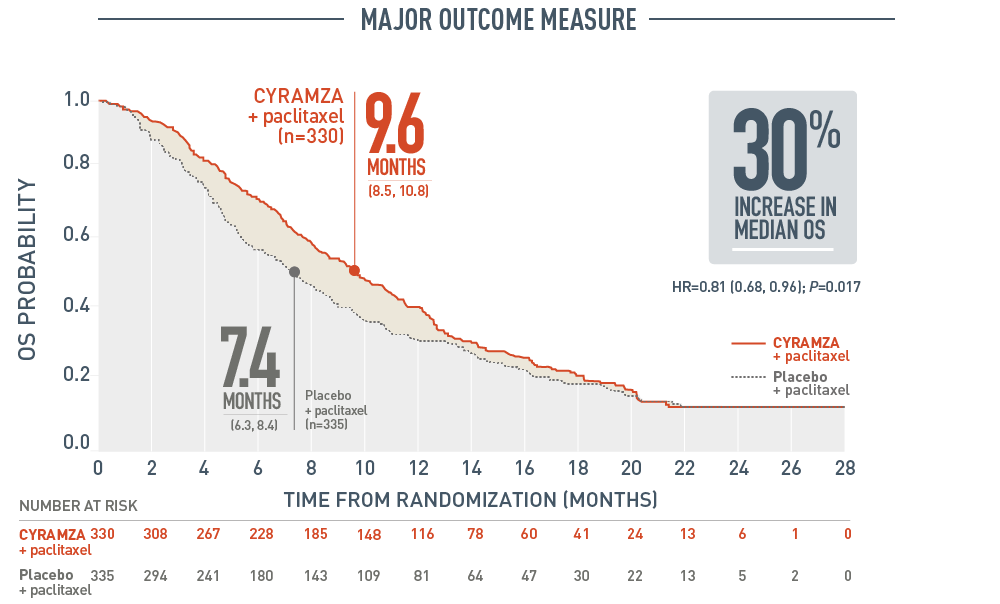
RAINBOW OS: MEDIAN – MONTHS (95% CI)1
The following three figures present efficacy data for the RAINBOW trial.
Overall survival (OS) was a major outcome measure of the RAINBOW trial. With CYRAMZA plus paclitaxel (n equals 330), median OS was 9.6 months with a 95 percent confidence interval of 8.5 to 10.8 months. With placebo plus paclitaxel (n equals 335), median OS was 7.4 months with a 95 percent confidence interval of 6.3 to 8.4 months. The hazard ratio was 0.81 with a 95 percent confidence interval of 0.68 to 0.96 and a P value of 0.017. The percentage of deaths at the time of the analysis was 78 percent (256 patients) in the CYRAMZA plus paclitaxel treatment arm and 78 percent (260 patients) in the placebo plus paclitaxel treatment arm of the trial.
- The percentage of deaths at the time of analysis was 78% (256 patients) and 78% (260 patients) in the CYRAMZA plus paclitaxel and placebo plus paclitaxel treatment arms, respectively.1
CI=confidence interval; ORR=overall response rate; OS=overall survival; PFS=progression-free survival.
SELECT IMPORTANT SAFETY INFORMATION
Impaired Wound Healing
- CYRAMZA has the potential to adversely affect wound healing. CYRAMZA has not been studied in patients with serious or non-healing wounds.
- Withhold CYRAMZA for 28 days prior to elective surgery. Do not administer CYRAMZA for at least 2 weeks following a major surgical procedure and until adequate wound healing. The safety of resumption of CYRAMZA after resolution of wound healing complications has not been established.
40% of patients on CYRAMZA + paclitaxel survived 1 year or longer2
What could more time mean to your patients?
Survival Rate % (95% CI)
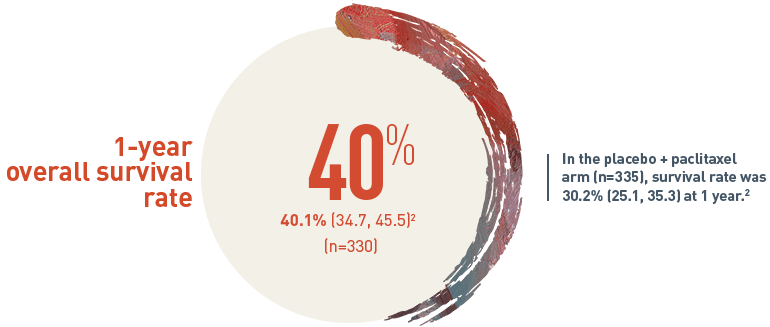
The 1-year overall survival (OS) rate for patients on CYRAMZA plus paclitaxel arm (n equals 330) was 40.1 percent with a 95 percent confidence interval of 34.7 to 45.5 percent. In the placebo plus paclitaxel treatment arm (n equals 335), the survival rate was 30.2 percent with a 95 percent confidence interval of 25.1 to 35.3 percent at one year.
Adding CYRAMZA to paclitaxel significantly increased PFS vs paclitaxel + placebo1
RAINBOW PFS: MEDIAN – MONTHS (95% CI)1,3
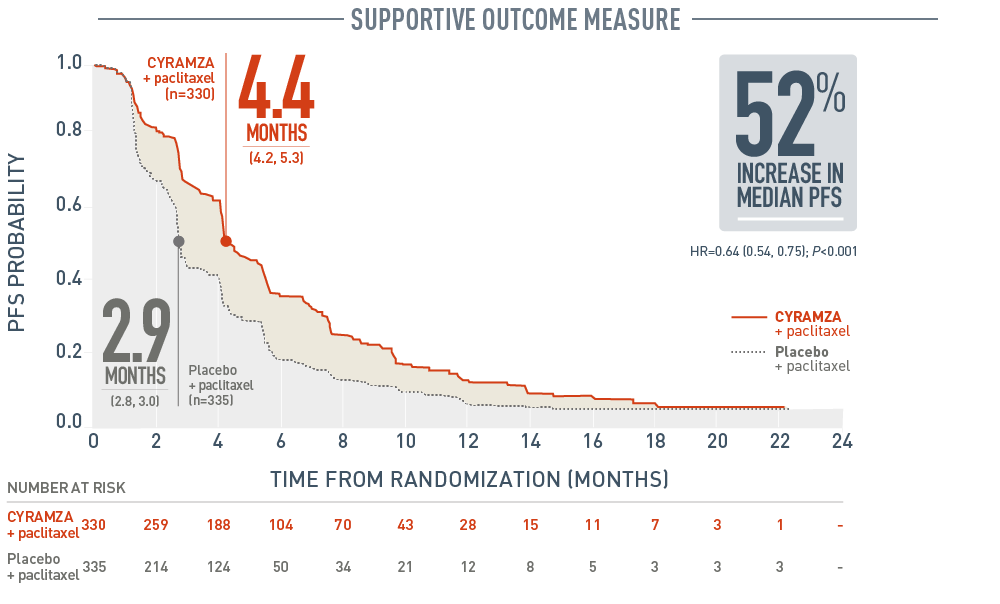
Progression-free survival (PFS) was a supportive outcome measure of the RAINBOW trial. With CYRAMZA plus paclitaxel (n equals 330), median PFS was 4.4 months with a 95 percent confidence interval of 4.2 to 5.3 months. With placebo plus paclitaxel (n equals 335), median PFS was 2.9 months with a 95 percent confidence interval of 2.8 to 3.0 months. The hazard ratio was 0.64 with a 95 percent confidence interval of 0.54 to 0.75 and a P value of less than 0.001. CYRAMZA plus paclitaxel achieved a 52 percent increase in median PFS when compared to placebo plus paclitaxel. The percentage of events at the time of analysis was 85 percent (279 patients) in the CYRAMZA plus paclitaxel treatment arm and 88 percent (296 patients) in the placebo plus paclitaxel treatment arm. In CYRAMZA-treated patients, 56 of 279 events were deaths. In the placebo-treated patients, 55 of 296 events were deaths.
- The percentage of events at the time of analysis was 85% (279 patients) and 88% (296 patients) in the CYRAMZA plus paclitaxel and placebo treatment arms, respectively1.
- 56 of 279 events in CYRAMZA-treated patients and 55 of 296 events in placebo-treated patients were deaths.
22% of patients on CYRAMZA + paclitaxel were progression free for 9 months or longer4
PFS Rate % (95% CI)4
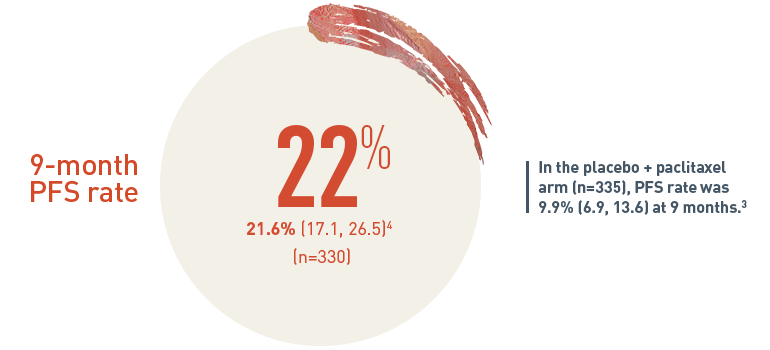
The 9-month progression-free survival (PFS) rate for patients on CYRAMZA plus paclitaxel arm (n equals 330) was 21.6 percent with a 95 percent confidence interval of 17.1 to 26.5 percent. In the placebo plus paclitaxel treatment arm (n equals 335), the PFS rate was 9.9 percent with a 95 percent confidence interval of 6.9 to 13.6 percent at nine months.
SELECT IMPORTANT SAFETY INFORMATION
Arterial Thromboembolic Events (ATEs)
- Serious, sometimes fatal, ATEs, including myocardial infarction, cardiac arrest, cerebrovascular accident, and cerebral ischemia, occurred across clinical trials. In 2137 patients with various cancers treated with CYRAMZA, the incidence of all Grade ATE was 1-3%. Grade 3-5 ATE incidence was <1- 2%.
- Permanently discontinue CYRAMZA in patients who experience an ATE
Adding CYRAMZA to paclitaxel nearly doubled the response vs paclitaxel alone1,3
RAINBOW ORR: PERCENT OF PATIENTS (95% CI)*1
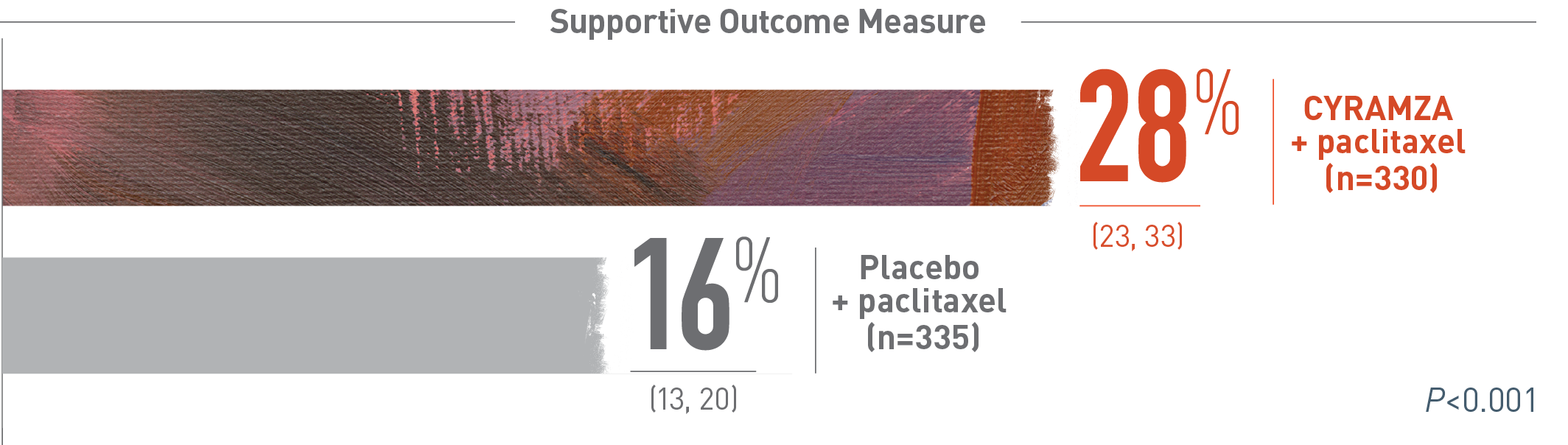
Overall response rate (ORR) was a supportive outcome measure of the RAINBOW trial. The following ORR results of the RAINBOW trial are presented. With CYRAMZA plus paclitaxel (n equals 330), ORR was 28 percent of patients with a 95 percent confidence interval of 23 to 33 percent. With placebo plus paclitaxel (n equals 335), ORR was 16 percent of patients with a 95 percent confidence interval of 13 to 20 percent. The P value was less than 0.001. ORR was defined as complete response plus partial response. Overall response rate does not include stable disease. Disease progression and tumor response were assessed by investigators in accordance with Response Evaluation Criteria in Solid Tumors (RECIST) 1.1.
*ORR was defined as complete plus partial response. Disease progression and tumor response were assessed by investigators in accordance with Response Evaluation Criteria in Solid Tumors (RECIST) 1.13
CI=confidence interval; ECOG=Eastern Cooperative Oncology Group; GEJ=gastroesophageal junction; HR=hazard ratio; ORR=overall response rate; OS=overall survival; PFS=progression-free survival; PS=performance status.
References
- CYRAMZA (ramucirumab) package insert. Indianapolis, IN: Eli Lilly and Company; 2021.
- Data on file, Eli Lilly and Company. ONC20170621b.
- Wilke H, Muro K, Van Cutsem E, et al; for the RAINBOW Study Group. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phased 3 trial. Lancet Oncol. 2014;15(11):1224-1235.
- Data on file, Eli Lilly and Company. ONC20170621a.
- Data on file, Eli Lilly and Company. ONC09302014b.
RAINBOW Disease Control Rate
CYRAMZA plus paclitaxel had an 80% Disease Control Rate* (DCR) in the RAINBOW trial1

Disease control rate (DCR) was an exploratory outcome measure of the RAINBOW trial. DCR was defined as complete response (CR) plus partial response (PR) plus stable disease. The following DCR results of the RAINBOW trial are presented in a bar chart. With CYRAMZA plus paclitaxel (n equals 330), DCR was 80 percent of patients with CR in 0.6 percent of patients (n equals 2), PR in 27.3 percent of patients (n equals 90), and stable disease in 52.1 percent of patients (n equals 172). With placebo plus paclitaxel (n equals 335), DCR was 64 percent of patients with CR in 0.3 percent of patients (n equals 1), PR in 15.8 percent of patients (n equals 53), and stable disease in 47.5 percent of patients (n equals 159). Overall response rate (ORR) was a pre-specified secondary efficacy endpoint in the RAINBOW trial, with DCR being an exploratory endpoint. Exploratory endpoints were not adequately powered or controlled for type 1 error, and treatment differences observed cannot be regarded as statistically significant.
ORR was a pre-specified secondary efficacy endpoint in RAINBOW, with DCR being an exploratory endpoint. Exploratory endpoints were not adequately powered or controlled for type 1 error, and treatment differences observed cannot be regarded as statistically significant.
*Disease control rate was defined as complete response plus partial response plus stable disease.
RAINBOW Depth of Response
Tumor Response* in Intent-to-Treat (ITT) Population2-4

The overall response rate (ORR) for CYRAMZA plus paclitaxel was 28 percent of patients (n equals 92 out of 330). Among the 92 patients with ORR, 74 percent (n equals 68) had tumor shrinkage of 40 percent or more. Sixty-eight patients in the CYRAMZA plus paclitaxel treatment arm and 38 patients in the placebo plus paclitaxel treatment arm had tumor shrinkage of 40 percent or more. Tumor shrinkage was calculated based on the post-baseline tumor measurements change from baseline on target lesions per Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Patients included in the assessment had measurable disease.
Depth of response was an exploratory outcome measure of the RAINBOW trial. With CYRAMZA plus paclitaxel, of the 92 patients included in the overall response rate (ORR), 68 patients (74 percent) had tumor shrinkage of 40 percent or more, 43 patients (47 percent) had tumor shrinkage 50 percent or more, 26 patients (28 percent) had tumor shrinkage 60 percent or more, 12 patients (13 percent) had tumor shrinkage 70 percent or more, and 8 patients (9 percent) had tumor shrinkage 80 percent or more. With placebo plus paclitaxel, of the 54 patients included in ORR, 38 patients (70 percent) had tumor shrinkage of 40 percent or more, 27 patients (50 percent) had tumor shrinkage 50 percent or more, 14 patients (26 percent) had tumor shrinkage 60 percent or more, 9 patients (17 percent) had tumor shrinkage 70 percent or more, and 5 patients (9 percent) had tumor shrinkage 80 percent or more. Overall response rate was a pre-specified secondary efficacy endpoint in the RAINBOW trial, with depth of response being an exploratory endpoint. Exploratory endpoints were not adequately powered or controlled for type 1 error, and treatment differences observed cannot be regarded as statistically significant.
ORR was a pre-specified secondary endpoint and depth of response was an exploratory endpoint. Exploratory endpoints were not adequately powered or controlled for type 1 error, and treatment differences observed cannot be regarded as statistically significant.
* Tumor shrinkage was calculated based on the post-baseline tumor measurements change from baseline on target lesions per Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 (Eisenhauer et al. 2009). Patients included in the assessment had measurable disease.
† 68 patients in the CYRAMZA + paclitaxel arm and 38 patients in the paclitaxel + placebo arm had tumor shrinkage of ≥40%. Patients who had non-measurable disease, no baseline tumor assessment, or no post-baseline tumor assessment were excluded from the tumor shrinkage analysis.
References
- Data on File. Lilly USA, LLC. DOF-RB-US-0063.
- Data on File, Lilly USA, LLC, DOF-RB-US-0062.
- Heinemann, V, Stintzing, S, Modest, DP, et al. Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur J Cancer. 2015;51(14):1927-1936.
- Lee, C-K, Kim, S-S, Park, S, et al. Depth of response is a significant predictor for long-term outcome in advanced gastric cancer patients treated with trastuzumab. Oncotarget. 2017;8(19):31169-31179.
SELECT IMPORTANT SAFETY INFORMATION
Hypertension
- An increased incidence of severe hypertension occurred in patients receiving CYRAMZA. In 1916 patients with various cancers treated with CYRAMZA, the incidence of all Grade hypertension ranged from 11-26%. Grade 3-5 hypertension incidence ranged from 6 15%
- Control hypertension prior to initiating treatment with CYRAMZA. Monitor blood pressure every two weeks or more frequently as indicated during treatment. Withhold CYRAMZA for severe hypertension until medically controlled. Permanently discontinue CYRAMZA for medically significant hypertension that cannot be controlled with antihypertensive therapy or in patients with hypertensive crisis or hypertensive encephalopathy.
Time to deterioration in Quality of Life (QoL) was evaluated using the patient-reported EORTC QLQ-C30, a secondary endpoint in the RAINBOW trial1
QoL Instrument
The EORTC QLQ-C30 is a self-administered, cancer-specific QoL instrument that assesses global health status, functioning, disease- and treatment-related symptoms, and financial difficulties.2 The QLQ-C30 was developed for use in all cancer types and is not specific to any stage, intervention, or line of therapy. The results are reported as 15 scales, each with a range of 0 to 100 points, according to the EORTC scoring manual.3
Assessment and Analysis Patients completed the QLQ-C30 at baseline, every 6 weeks following the first dose of study therapy until documentation of progressive disease, and at the end-of-therapy visit.
For each scale, time to deterioration was defined as the time from randomization to the first deterioration event, which was pre-specified as a worsening of ≥10 points from baseline. Time to deterioration was compared between arms using an unstratified Cox proportional hazards model. The analysis was conducted in the intent-to-treat (ITT) population
Although a change of ≥10 points has been reported as meaningful for the QLQ-C304, the magnitudes of change for each of the 15 scales in patients with advanced/metastatic gastric cancer may differ.
Results
At baseline, 98% of patients in each arm completed the QLQ-C30. Of the patients still receiving treatment in the study at weeks 6, 12, and 18, >83% of patients4 in each arm completed the QLQ-C30. Baseline scores were similar between arms.
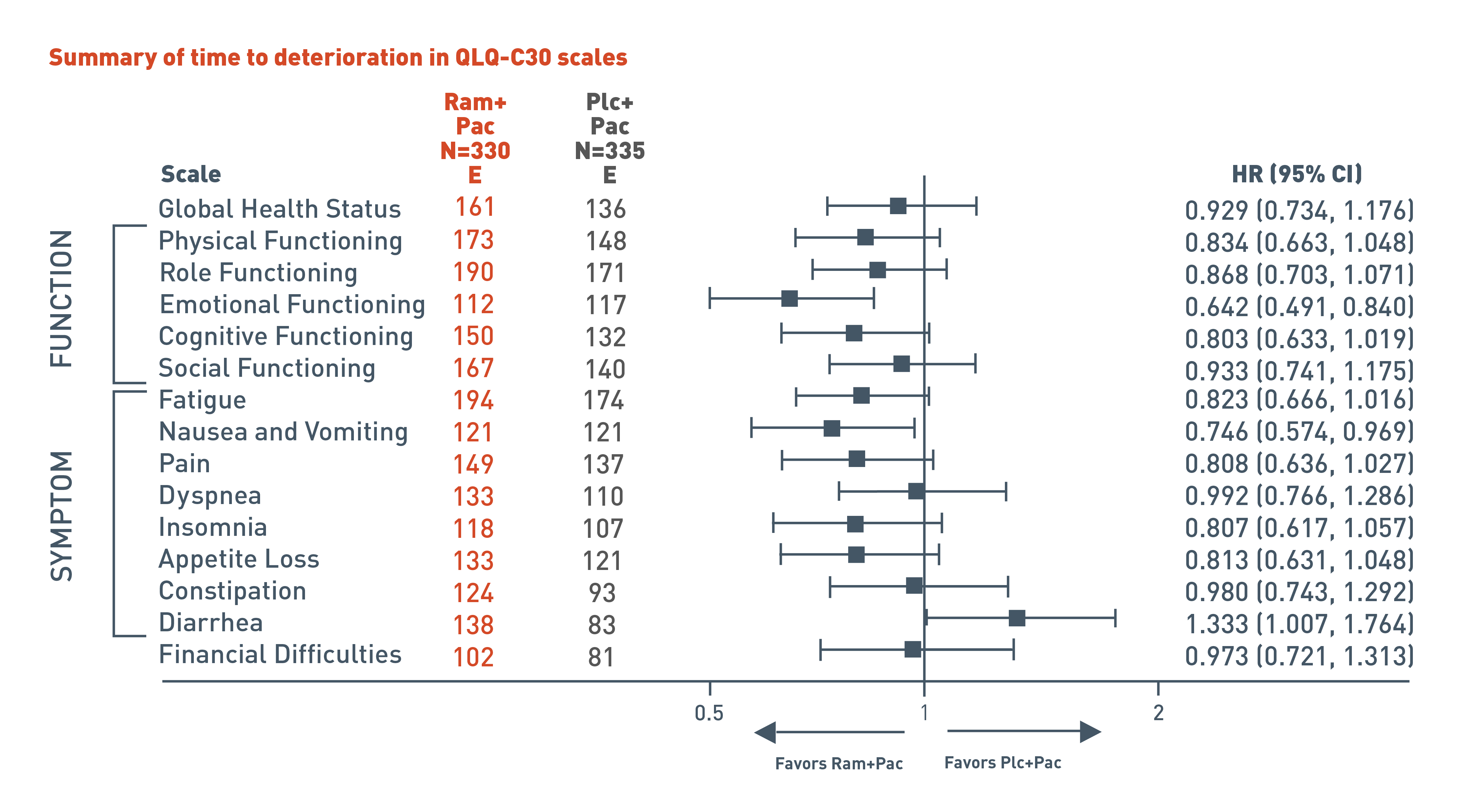
The RAINBOW trial evaluated time to deterioration analyses in global health status and quality of life using patient reported European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30. In addition to global health status, the QLQ-C30 incorporates five multi-item functional scales (physical, role, cognitive, emotional, and social); three multi-item symptom scales (fatigue, pain, and nausea and vomiting); and several single-item symptom measures.
The following results for the pre-specified secondary endpoint of the RAINBOW trial are presented in a forest plot for patients in the CYRAMZA plus paclitaxel arm (n equals 330) and in the placebo plus paclitaxel arm (n equals 335).
For global health status, 161 events were reported in the CYRAMZA plus paclitaxel arm and 136 events in the placebo plus paclitaxel arm. The hazard ratio was 0.929 with a 95 percent confidence interval 0.734 to 1.176.
For physical functioning scale, 173 events were reported in the CYRAMZA plus paclitaxel arm and 148 events in the placebo plus paclitaxel arm. The hazard ratio was 0.834 with a 95 percent confidence interval 0.663 to 1.048.
For role functioning scale, 190 events were reported in the CYRAMZA plus paclitaxel arm and 171 events in the placebo plus paclitaxel arm. The hazard ratio was 0.868 with a 95 percent confidence interval 0.703 to 1.071.
For emotional functioning scale, 112 events were reported in the CYRAMZA plus paclitaxel arm and 117 events in the placebo plus paclitaxel arm. The CYRAMZA plus paclitaxel group was favored. The hazard ratio was 0.642 with a 95 percent confidence interval 0.491 to 0.840.
For cognitive functioning scale, 150 events were reported in the CYRAMZA plus paclitaxel arm and 132 events in the placebo plus paclitaxel arm. The hazard ratio was 0.803 with a 95 percent confidence interval 0.633 to 1.019.
For social functioning scale, 167 events were reported in the CYRAMZA plus paclitaxel arm and 140 events in the placebo plus paclitaxel arm. The hazard ratio was 0.933 with a 95 percent confidence interval 0.741 to 1.175.
For the fatigue symptom scale, 194 events were reported in the CYRAMZA plus paclitaxel arm and 174 events in the placebo plus paclitaxel arm. The hazard ratio was 0.823 with a 95 percent confidence interval 0.666 to 1.016.
For the nausea and vomiting symptom scale, 121 events were reported in the CYRAMZA plus paclitaxel arm and 121 events in the placebo plus paclitaxel arm. The hazard ratio was 0.746 with a 95 percent confidence interval 0.574 to 0.969.
For the pain symptom scale, 149 events were reported in the CYRAMZA plus paclitaxel arm and 137 events in the placebo plus paclitaxel arm. The hazard ratio was 0.808 with a 95 percent confidence interval 0.636 to 1.027.
For the dyspnea symptom scale, 133 events were reported in the CYRAMZA plus paclitaxel arm and 110 events in the placebo plus paclitaxel arm. The hazard ratio was 0.992 with a 95 percent confidence interval 0.766 to 1.286.
For the insomnia symptom scale, 118 events were reported in the CYRAMZA plus paclitaxel arm and 107 events in the placebo plus paclitaxel arm. The hazard ratio was 0.807 with a 95 percent confidence interval 0.617 to 1.057.
For the appetite loss symptom scale, 133 events were reported in the CYRAMZA plus paclitaxel arm and 121 events in the placebo plus paclitaxel arm. The hazard ratio was 0.813 with a 95 percent confidence interval 0.631 to 1.048.
For the constipation symptom scale, 124 events were reported in the CYRAMZA plus paclitaxel arm and 93 events in the placebo plus paclitaxel arm. The hazard ratio was 0.980 with a 95 percent confidence interval 0.743 to 1.292.
For the diarrhea symptom scale, 138 events were reported in the CYRAMZA plus paclitaxel arm and 83 events in the placebo plus paclitaxel arm. The placebo plus paclitaxel group was favored. The hazard ratio was 1.333 with a 95 percent confidence interval 1.007 to 1.764.
For the financial difficulties scale, 102 events were reported in the CYRAMZA plus paclitaxel arm and 81 events in the placebo plus paclitaxel arm. The hazard ratio was 0.973 with a 95 percent confidence interval 0.721 to 1.313.
Time to deterioration analyses in quality of life was evaluated using the patient reported EORTC QLQ-C30, a pre-specified secondary endpoint in the RAINBOW trial. This was not a gated endpoint and no specific hypothesis was tested, so results should only be interpreted descriptively. The analyses were not adequately powered to demonstrate any statistically significant effects and no adjustments were made for multiplicity.
Adverse events reported in clinical trials should not be compared with patient-reported outcomes.
CI=confidence interval; E=number of events; EORTC=European Organisation for Research and Treatment Cancer; HR=hazard ratio; N=randomized patients; Pac=Paclitaxel; Plc=Placebo; Ram=Ramucirumab.
References
- Al-Batran SE, Van Cutsem E, Oh SC, et al. Quality-of-life and performance status results from the phase III RAINBOW study of ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated gastric or gastroesophageal junction adenocarcinoma. Ann Oncol. 2016;27(4):673-679.
- Aaronson NK, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-76.
- Fayers P, Aaronson NK, Bjordal K, Curran D, Broenvold M, on behalf of the EORTC Quality of Life Study Group. EORTC QLQ-C30 Scoring Manual. 3rd ed. Brussels (Belgium): EORTC Quality of Life Group, 2001.
- Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139-144.