
The REACH Program comprised 2 global phase III trials (REACH and REACH-2) that identified AFP as a biomarker for treatment selection for CYRAMZA in advanced HCC1,2,4,5
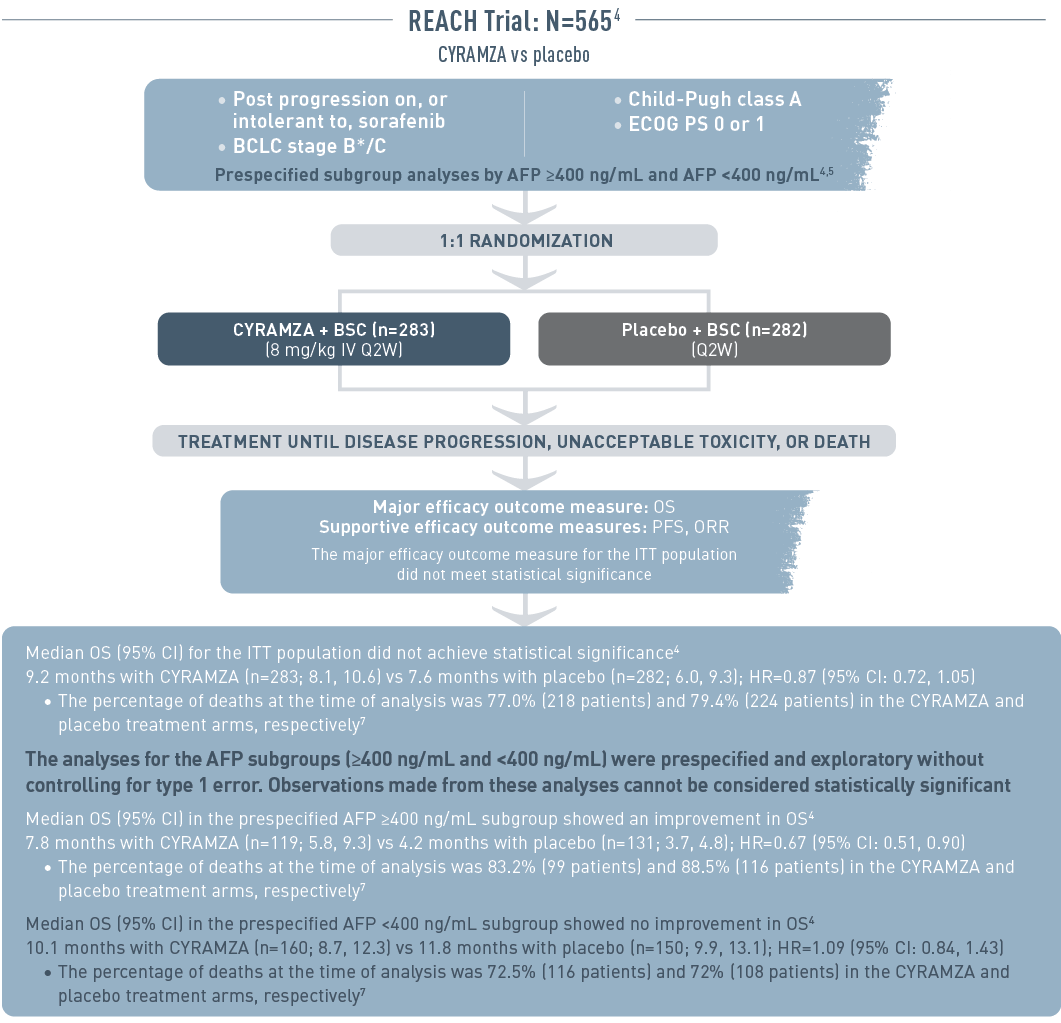
The REACH trial (total N equals 565) included patients with advanced hepatocellular carcinoma who had progressed on or were intolerant to sorafenib therapy. The patient population had Barcelona Clinic Liver Cancer stage B (and no longer amenable to locoregional therapy) or C disease, Child-Pugh class A liver disease, and an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1. Patient subgroup analysis was prespecified by alpha-fetoprotein (AFP) of 400 nanograms per milliliter or higher or less than 400 nanograms per milliliter. Patients were randomized 1 to 1 to receive intravenous CYRAMZA 8 milligrams per kilogram plus best supportive cancer (BSC), (n equals 283), or placebo plus BSC (n equals 282) every 2 weeks. Treatment was continued until disease progression, unacceptable toxicity, or death. The major efficacy outcome measure was overall survival (OS). Supportive efficacy outcome measures included progression-free survival (PFS) and overall response rate (ORR). The major efficacy outcome for the intent-to-treat (ITT) population did not meet statistical significance.
Median OS for the ITT population did not achieve statistical significance. Median OS with CYRAMZA (n equals 283) was 9.2 months with a 95 percent confidence interval of 8.1 to 10.6 months vs 7.6 months with placebo (n equals 282) with a 95 percent confidence interval of 6.0 to 9.3 months. The hazard ratio was 0.87 with a 95 percent confidence interval of 0.72 to 1.05. The percentage of deaths at the time of analysis was 77.0 percent (218 patients) and 79.4 percent (224 patients) in the CYRAMZA and placebo treatment arms, respectively.
The analyses for the AFP subgroups (400 nanograms per milliliter or higher and less than 400 nanograms per milliliter) were prespecified and exploratory without controlling for type 1 error. Observations made from these analyses cannot be considered statistically significant.
Median OS for the prespecified subgroup with AFP 400 nanograms per milliliter or higher showed an improvement in OS. Median OS with CYRAMZA (n equals 119) was 7.8 months with a 95 percent confidence interval of 5.8 to 9.3 months vs 4.2 months with placebo (n equals 131) with a 95 percent confidence interval of 3.7 to 4.8 months. The hazard ratio was 0.67 with a 95 percent confidence interval of 0.51 to 0.90. The percentage of deaths at the time of analysis was 83.2 percent (99 patients) and 88.5 percent (116 patients) in the CYRAMZA and placebo treatment arms, respectively.
Median OS for the prespecified subgroup with AFP less than 400 nanograms per milliliter showed no improvement in OS. Median OS with CYRAMZA (n equals 160) was 10.1 months with a 95 percent confidence interval of 8.7 to 12.3 months vs 11.8 months with placebo (n equals 150) with a 95 percent confidence interval of 9.9 to 13.1 months. The hazard ratio was 1.09 with a 95 percent confidence interval of 0.84 to 1.43. The percentage of deaths at the time of analysis was 72.5 percent (116 patients) and 72 percent (108 patients) in the CYRAMZA and placebo treatment arms, respectively.
* And no longer amenable to locoregional therapy.
BCLC=Barcelona Clinic Liver Cancer; BSC=best supportive care; CL=confidence interval; ECOG=Eastern Cooperative Oncology Group; HR=hazard ratio; ITT=intent-to-treat; IV=intravenous; ORR=overall response rate; OS=overall survival; PFS=progression-free survival; Q2W=every 2 weeks.
The REACH‑2 trial was purposefully designed to evaluate AFP as a biomarker for treatment selection1,2
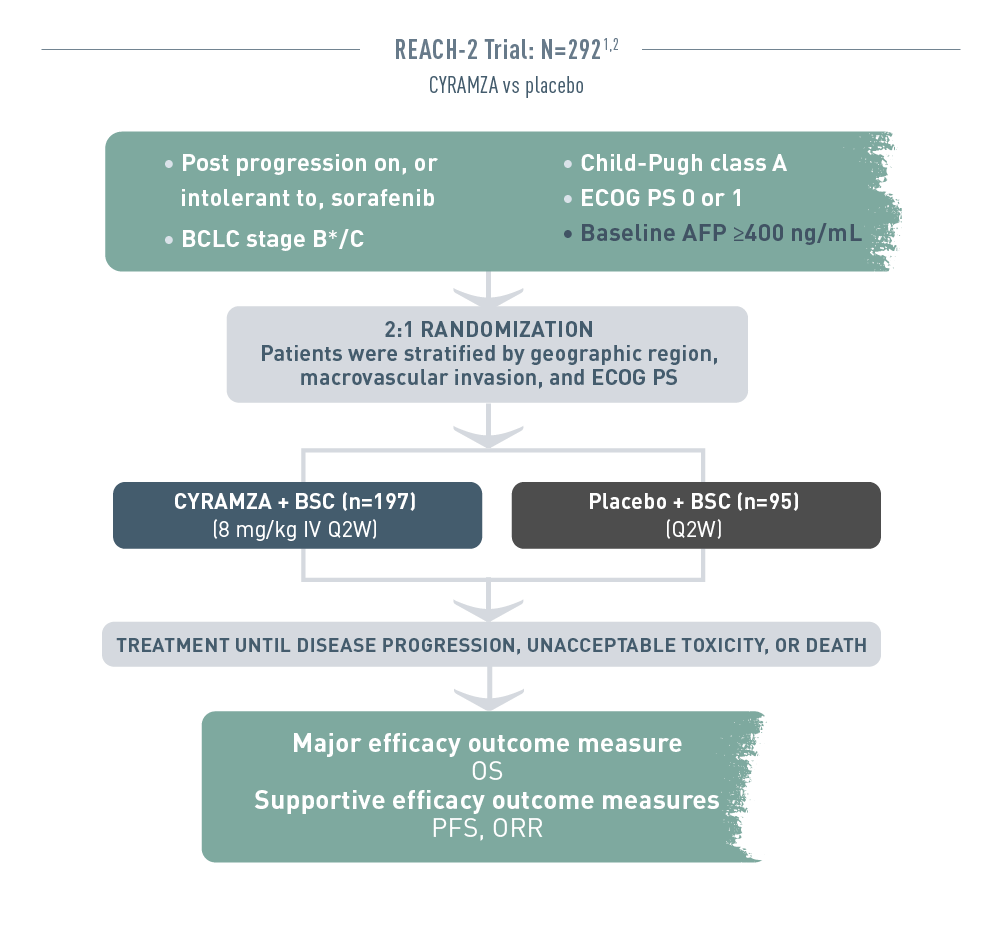
The REACH 2 trial (total N equals 292) included patients with advanced hepatocellular carcinoma who had progressed on or were intolerant to sorafenib therapy. The patient population had Barcelona Clinic Liver Cancer stage B (and no longer amenable to locoregional therapy) or C disease, Child-Pugh class A liver disease, an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, and a baseline alpha-fetoprotein (AFP) of 400 nanograms per milliliter or higher. Patients were randomized 2 to 1 to receive intravenous CYRAMZA 8 milligrams per kilogram plus best supportive cancer (BSC), (n equals 197), or placebo plus BSC (n equals 95) every 2 weeks. Patients were stratified by geographic region, macrovascular invasion, and ECOG PS. Treatment was continued until disease progression, unacceptable toxicity, or death. The major efficacy outcome measure was overall survival (OS). Supportive efficacy outcome measures included progression-free survival (PFS) and overall response rate (ORR).
* And no longer amenable to locoregional therapy.
SELECT IMPORTANT SAFETY INFORMATION
Gastrointestinal Perforations
- CYRAMZA can increase the risk of gastrointestinal perforation, a potentially fatal event. In 2137 patients with various cancers treated with CYRAMZA, the incidence of all Grade and Grade 3-5 gastrointestinal perforations ranged from <1-2%.
- Permanently discontinue CYRAMZA in patients who experience a gastrointestinal perforation.
See efficacy data for your AFP-High patients
Demographics and Baseline Characteristics in REACH‑23

The following demographic and baseline characteristics are for the REACH 2 trial.
For patients in the CYRAMZA treatment arm (n equals 197), 78 percent were male, the median age was 64, 57 percent had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0, 51 percent were from the Americas, Europe, Israel, and Australia, 28 percent were from Asia (excluding Japan), 21 percent were from Japan, 62 percent had a Child-Pugh score of A-5, 17 percent had Barcelona Clinic Liver Cancer (BCLC) stage B disease, 84 percent discontinued sorafenib due to progressive disease, 16 percent discontinued sorafenib due to intolerance, 36 percent had hepatitis B, 24 percent had hepatitis C, 24 percent had significant alcohol use, 36 percent had macrovascular invasion, and 72 percent had extrahepatic spread. The median baseline alpha-fetoprotein (AFP) value was 3920.0 with concentrations ranging from a minimum of 408.0 to a maximum of 230500.0.
For patients in the placebo treatment arm (n equals 95), 83 percent were male, the median age was 64, 58 percent had an ECOG PS of 0, 53 percent were from the Americas, Europe, Israel, and Australia, 28 percent were from Asia (excluding Japan), 19 percent were from Japan, 57 percent had a Child-Pugh score of A-5, 21 percent had BCLC stage B disease, 80 percent discontinued sorafenib due to progressive disease, 20 percent discontinued sorafenib due to intolerance, 38 percent had hepatitis B, 30 percent had hepatitis C, 22 percent had significant alcohol use, 35 percent had macrovascular invasion, and 74 percent had extrahepatic spread. The median baseline AFP value was 2741.0 with concentrations ranging from a minimum of 419.0 to a maximum of 473163.0.
ECOG PS, macrovascular invasion, and baseline AFP were prognostic in a stepwise Cox regression.
† Prognostic in a stepwise Cox regression.3
AFP=alpha-fetoprotein; BCLC=Barcelona Clinic Liver Cancer; BSC=best supportive care; ECOG=Eastern Cooperative Oncology Group; HCC=hepatocellular carcinoma; IV=intravenous; ORR=overall response rate; OS=overall survival; PFS=progression-free survival; PS=performance status; Q2W=every 2 weeks.
References
- CYRAMZA (ramucirumab) package insert. Indianapolis, IN: Eli Lilly and Company; 2021.
- Zhu AX, Kang Y-K, Yen C-J, et al; for REACH‑2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH‑2): a randomised, double-blind, placebo-controlled, phase 3 trial [published online January 18, 2019]. Lancet Oncol. doi:10.1016/S1470-2045(18)30937-9.
- Zhu AX, Finn RS, Galle PR, et al. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated alpha-fetoprotein (AFP) following first-line sorafenib: pooled efficacy and safety across two global randomized phase 3 studies (REACH‑2 and REACH). Poster presented at: ESMO 20th World Congress on Gastrointestinal Cancer; June 20-23, 2018; Barcelona, Spain.
- Zhu AX, Park JO, Ryoo B-Y, et al; REACH Trial Investigators. Ramucirumab versus placebo as second line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859- 870.
- Supplement to: Zhu AX, Park JO, Ryoo B-Y, et al; REACH Trial Investigators. Ramucirumab versus placebo as second line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859-870
- Zhu AX, Kang YK, Yen C-J, et al. REACH-2: A randomized, double-blind, placebo-controlled phase 3 study of ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma and elevated baseline alpha-fetoprotein following first-line sorafenib. J Clin Oncol. 2018; 36(suppl; abstr 4003).
- Zhu AX et al. J Clin Oncol. 33, 2015 (suppl 3; abstr 232)