
Is it time for a change in your treatment strategy?
Treating your patients with mCRC who rapidly progressed* on first-line therapy
mCRC Clinical Case Presentation by Dr. Braiteh
Fadi S. Braiteh, MD
Medical Oncologist, Clinical Investigator, Medical Director, Translational Oncology (Phase I) and GI Malignancies Programs
Comprehensive
Cancer Centers of Nevada
Clinical Associate Professor of Medicine, University of Nevada, Las Vegas
Las Vegas, Nevada
CYRAMZA, in combination with FOLFIRI (irinotecan, folinic acid, and fluorouracil), is indicated for the treatment of patients with metastatic colorectal cancer (mCRC) with disease progression on or after prior therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine.
SELECT IMPORTANT SAFETY INFORMATION
Impaired Wound Healing
- CYRAMZA has the potential to adversely affect wound healing. CYRAMZA has not been studied in patients with serious or non-healing wounds.
- Withhold CYRAMZA for 28 days prior to elective surgery. Do not administer CYRAMZA for at least 2 weeks following a major surgical procedure and until adequate wound healing. The safety of resumption of CYRAMZA after resolution of wound healing complications has not been established.
Patient Presentation
Medical History
- No personal history for disease and no family history of malignancies
- Healthy individual
Social History
- Stay-at-home mother
- 4 children
- No history of tobacco or alcohol use
History of Present Illness
- The patient, Diane†, is a 50-year-old female who presented with left lower quadrant pain
- A colonoscopy revealed a completely obstructive sigmoid mass
- Biopsy confirmed moderately differentiated adenocarcinoma
- CT scan of the chest, abdomen, and pelvis revealed multiple metastases in the lymph nodes and spleen
- Biopsy of the liver lesion confirmed metastatic adenocarcinoma of the colon
- The patient underwent palliative surgery consisting of a diverting colostomy due to the obstructive nature of the sigmoid mass
- Molecular testing revealed KRAS exon 2 codon 13 mutation, APC and TP53 mutations, and microsatellite stable status
† Hypothetical Patient
Treatment History

The following treatment history for a hypothetical patient is presented graphically.
Initial treatment is started and consists of first-line (1L) bevacizumab plus modified folinic acid, fluorouracil, oxaliplatin (mFOLFOX6). Bevacizumab was held for first cycle due to surgery.
Cycle 4: A computerized tomography (CT) scan revealed improvement in liver lesions.
Cycle 8: A CT scan revealed further improvement in liver lesions and resolution of lymph node metastases. Oxaliplatin was dropped as preplanned.
Cycle 12: Treatment is well tolerated, without the need of a dose adjustment. Patient developed right lower quadrant pain.
After cycle 12, progression is confirmed. A CT scan revealed a right ovarian mass. Patient undergoes a right oophorectomy due to pain and ovarian torsion. Pathology confirmed metastatic adenocarcinoma of the colon primary. The patient's Eastern Cooperative Oncology Group performance status (ECOG PS) is 0.
Patient goes onto second-line (2L) treatment.
Patient Attitude
- Patient recovered well from surgery; she presented to the medical oncologist aware of her situation and in the face of adversity, she remains determined
* Defined as <6 months on prior therapy
This is a hypothetical patient case based on the author’s clinical experience with CYRAMZA in combination with FOLFIRI for mCRC.
This clinical case presentation has been sponsored by Eli Lilly and Company.
1L=first-line; 2L=second-line; CT=computerized tomography; ECOG=Eastern Cooperative Oncology Group; FOLFOX=folinic acid, fluorouracil, oxaliplatin; PS=performance status
mCRC=metastatic colorectal cancer; KRAS=Kirsten rat sarcoma; FOLFORI=irinotecan, folinic acid, and fluorouracil; APC=adenomatous polyposis coli.
Why Dr. Braiteh Chose CYRAMZA + FOLFIRI
Treatment Plan
- CYRAMZA + FOLFIRI;
- CYRAMZA was held for the first 2 cycles due to recent visceral surgery
My rationale for considering CYRAMZA as an option for this patient
- She initially responded to bevacizumab + FOLFOX but progressed after 12 cycles (6 months)
- Because she rapidly progressed*, she may benefit from a different approach
- The RAISE study included patients who rapidly progressed* (23% of study arm) as well as whose tumors harbored KRAS mutations (50% of study arm)1-3
- She is determined to continue a combination treatment
- CYRAMZA has demonstrated an OS and PFS improvement when added to FOLFIRI in the RAISE study1
- In patients with rapidly progressing disease*, the OS and PFS improvement was consistent with the RAISE ITT1-3
Response to CYRAMZA + FOLFIRI
- After 6 cycles, PET/CT scan revealed no growth in metastases
- ECOG PS 0
How would you treat a patient like Diane?
How does it compare with Dr. Braiteh’s management of Diane?
* Defined as <6 months on prior therapy.
Consider a Change to CYRAMZA + FOLFIRI for Your Patients Like Diane*
Statistically Significant Improvement in OS in the ITT Population; Consistent Results Within Prespecified and Exploratory Subgroup Analyses1-2,4
OS: Median (95% CI)
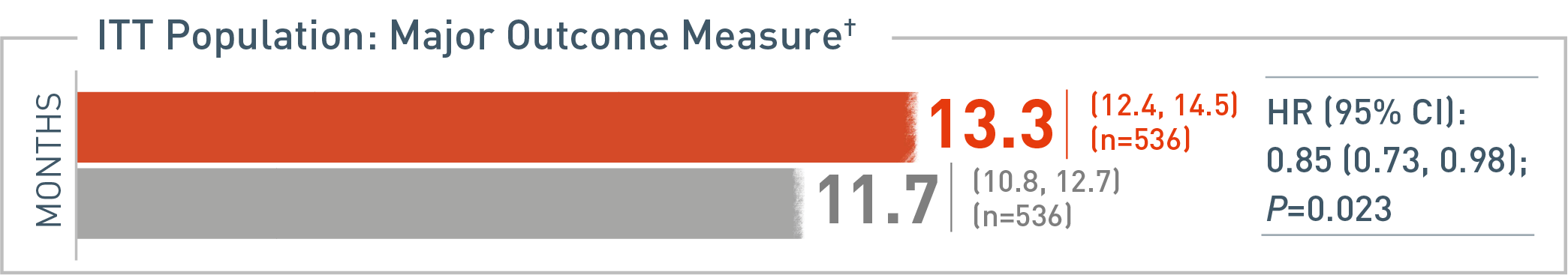
The following results for the intent-to-treat patient population with metastatic colorectal cancer (mCRC) in the RAISE trial are presented.
Overall survival (OS) was the major efficacy outcome measure of the RAISE trial. With CYRAMZA plus irinotecan, folinic acid, and fluorouracil (FOLFIRI), (n equals 536), median OS was 13.3 months with a 95 percent confidence interval of 12.4 to 14.5 months. With placebo plus FOLFIRI (n equals 536) median OS was 11.7 months with a 95 percent confidence interval of 10.8 to 12.7 months. The hazard ratio was 0.85 with a 95 percent confidence interval of 0.73 to 0.98 and the P value equaled 0.023.
RAISE Subgroup OS
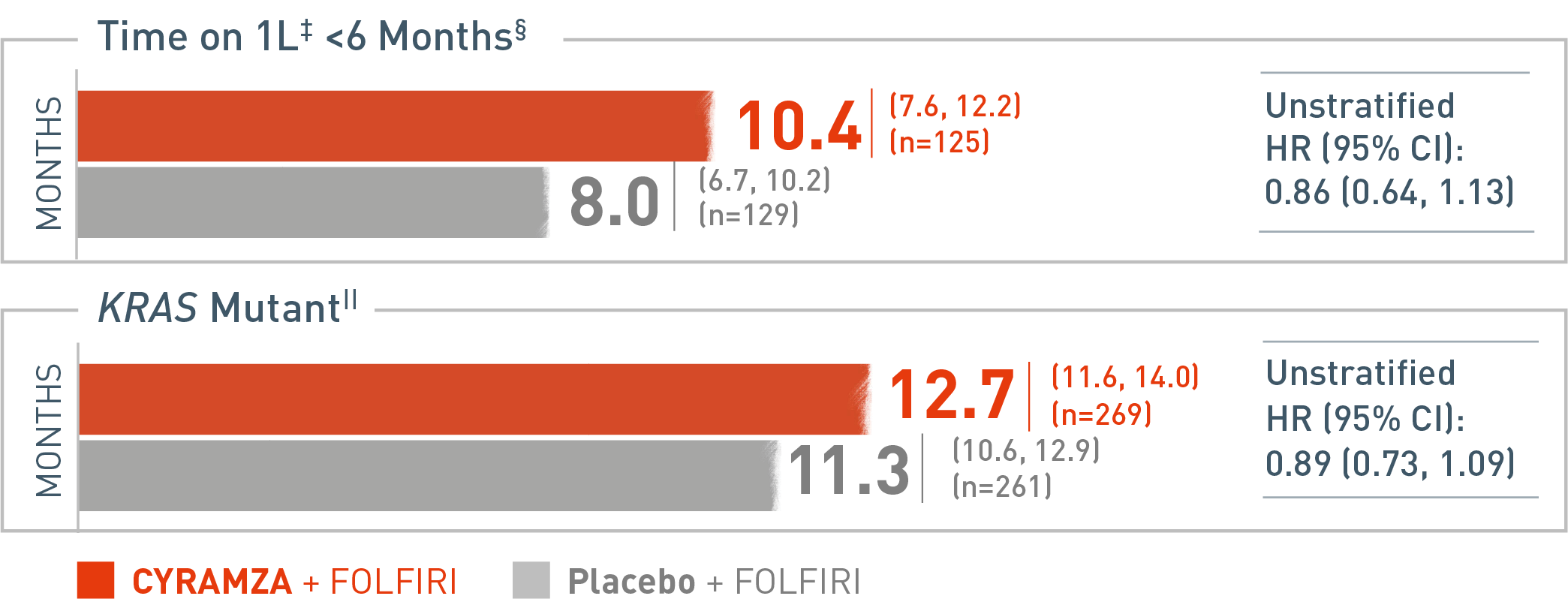
Overall survival (OS) was assessed in a subgroup of patients whose time on first-line (1L) treatment was less than six months. The following results are presented. With CYRAMZA plus irinotecan, folinic acid, and fluorouracil (FOLFIRI), (n equals 125), median OS was 10.4 months with a 95 percent confidence interval of 7.6 to 12.2 months. With placebo plus FOLFIRI (n equals 129) median OS was 8.0 months with a 95 percent confidence interval of 6.7 to 10.2 months. The hazard ratio was 0.86 with a 95 percent confidence interval of 0.64 to 1.13.
Time on 1L refers to disease progression on first-line therapy. Based on case report form (CRF) data if present, or interactive voice response system value if CRF data were missing for the parameter. Overall survival (OS) was assessed in a subgroup of patients whose tumors carry a Kirsten rat sarcoma (Kras) mutation. The following results are presented. With CYRAMZA plus irinotecan, folinic acid, and fluorouracil (FOLFIRI), (n equals 269), median OS was 12.7 months with a 95 percent confidence interval of 11.6 to 14.0 months. With placebo plus FOLFIRI (n equals 261) median OS was 11.3 months with a 95 percent confidence interval of 10.6 to 12.9 months. The hazard ratio was 0.89 with a 95 percent confidence interval of 0.73 to 1.09.
The RAISE trial was not adequately powered, nor error-controlled, for subgroup analyses. Treatment differences observed in these subgroups cannot be regarded as statistically significant. The analyses described here were pre-specified and exploratory.
* Hypothetical patient.
† The percentage of deaths at the time of analysis was 69% (372 patients) and 74% (397 patients) in the CYRAMZA plus FOLFIRI and placebo plus FOLFIRI arms, respectively.1
‡ Time on 1L refers to time to disease progression on first-line therapy.
§ For patients with 1L progression <6 months, the percentage of deaths at the time of analysis in the CYRAMZA plus FOLFIRI arm was 73.6% (92 patients) and 79.1% (102 patients) in the placebo plus FOLFIRI arm.4
|| For KRAS mutant patients, the percentage of events at the time of analysis in the CYRAMZA plus FOLFIRI arm was 72.9% (196 patients) and 75.9% (198 patients) in the placebo plus FOLFIRI arm.4
Supportive Outcome Measure
Median PFS in the ITT population was 5.7 months (95% CI: 5.5, 6.2) for CYRAMZA + FOLFIRI (n=536) vs 4.5 months (95% CI: 4.2, 5.4) for placebo + FOLFIRI (n=536); HR=0.79 (95% CI: 0.70, 0.90); P<0.001¶1 The treatment effect was consistent across prespecified stratification factors.
- The percentage of events at the time of analysis was 89% (476 patients) and 92% (494 patients) in the CYRAMZA plus FOLFIRI and placebo plus FOLFIRI arms, respectively. 73 of 476 events in the CYRAMZA-treated patients and 64 of 494 events in the placebo-treated patients were deaths.1
RAISE Subgroup PFS
Median PFS with CYRAMZA + FOLFIRI (n=125) in patients on first line less than 6* months was 5.2 months (95% CI: 4.0, 6.2) vs 2.9 months (95% CI: 2.7, 4.1) with placebo + FOLFIRI (n=129) (unstratified HR=0.68 95% CI: 0.52, 0.89).2,4
- The percentage of events at the time of analysis was 89% (111 patients) and 91% (117 patients) in the CYRAMZA + FOLFIRI and placebo + FOLFIRI arms, respectively4
Patients with KRAS mutant status (unstratified HR =0.77, 95% CI: 0.70, 1.00): Median PFS was 5.6 months (5.1, 6.8) with CYRAMZA + FOLFIRI (n=269) vs. 4.3 months (4.1, 5.4) with placebo + FOLFIRI (n=261)2,4
- For KRAS mutant patients, the percentage of events at the time of analysis was 90% (243 patients) and 91% (237 patients) in the CYRAMZA + FOLFIRI and placebo + FOLFIRI arms, respectively4
The RAISE trial was not adequately powered, nor error-controlled, for subgroup analyses. Treatment differences observed in these subgroups cannot be regarded as statistically significant. The analyses described here were pre-specified and exploratory.
SELECT IMPORTANT SAFETY INFORMATION
The labeling for CYRAMZA contains warnings and precautions for hemorrhage and GI hemorrhage, including severe and sometimes fatal events; gastrointestinal (GI) perforations, a potentially fatal event; impaired wound healing; arterial thromboembolic events (ATEs), including serious and sometimes fatal events; hypertension; infusionrelated reactions (IRR), including severe and sometimes fatal events; worsening of pre-existing hepatic impairment; posterior reversible encephalopathy syndrome (PRES), including fatal events; proteinuria including nephrotic syndrome; thyroid dysfunction; and embryo-fetal toxicity. CYRAMZA should be permanently discontinued in patients who experience severe bleeding, a GI perforation, an ATE, uncontrolled hypertension, severe IRR, PRES, or urine protein >3 grams/24 h or nephrotic syndrome.
In RAISE, the most common adverse reactions (all Grades) observed in CYRAMZA with FOLFIRI-treated patients at a rate of ≥30% and ≥2% higher than placebo with FOLFIRI were diarrhea (60% vs 51%), neutropenia (59% vs 46%), decreased appetite (37% vs 27%), epistaxis (33% vs 15%), and stomatitis (31% vs 21%). The most common serious adverse reactions with CYRAMZA with FOLFIRI were diarrhea (3.6%), intestinal obstruction (3.0%), and febrile neutropenia (2.8%); 20% of patients treated with CYRAMZA with FOLFIRI received granulocyte colony-stimulating factors.
RAISE Trial Design (N=1072) The phase III RAISE trial evaluated the efficacy and safety of CYRAMZA plus FOLFIRI vs placebo plus FOLFIRI in patients with mCRC with disease progression on or after prior therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine. Major efficacy outcome measure was overall survival. Supportive efficacy outcome measure was progression-free survival. All patients were required to have Eastern Cooperative Oncology Group performance status 0 or 1. Patients were stratified by geographic region, KRAS mutation status, and time to disease progression after the beginning of first-line treatment (<6 months vs ≥6 months). Patients were randomized 1:1 (N=1072) to receive either CYRAMZA 8 mg/kg or placebo, in combination with FOLFIRI every 14 days.1
Based on case report form (CRF) data if present, or interactive voice response system value if CRF data were missing for the parameter.
CI=confidence interval; HR=hazard ratio ITT=intent to treat. PET=positron emission tomography
For your patients with mCRC whose disease rapidly progressed* on 1L therapy1

References
- CYRAMZA (ramucirumab) package insert. Indianapolis, IN: Eli Lilly and Company; 2021.
- Obermannová R, Van Cutsem E, Yoshino T, et al. Subgroup analysis in RAISE: a randomized, doubleblind phase III study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab or placebo in patients with metastatic colorectal carcinoma progression. Ann Oncol. 2016;27(11):2082-2090.
- Data on File, Lilly USA, LLC, DOF-RB-US-0030.
- Data on File, Lilly USA, LLC, DOF-RB-US-0040.