
Metastatic Non-Small Cell Lung Cancer
REVEL trial: Overall response rate (ORR)
Statistically significant improvement in ORR in the ITT population1
Half of patients who received CYRAMZA + docetaxel achieved an estimated PFS of 4.5 months or longer vs 3.0 months or longer with docetaxel alone1
REVEL ORR: Percentage of Patients (95% CI)1*
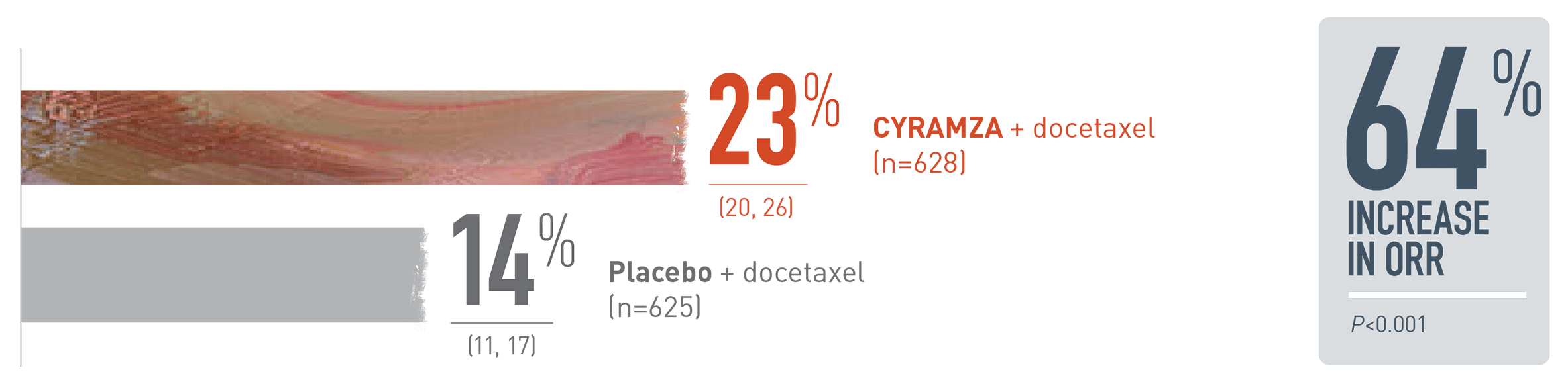
Overall response rate (ORR) was a supportive outcome measure of the REVEL trial.
The following ORR results for patients with metastatic non-small cell lung cancer (mNSCLC) in the REVEL trial are presented in a bar chart. With CYRAMZA plus docetaxel in the intent-to-treat (ITT) population (n equals 628), ORR was 23 percent of patients with a 95 percent confidence interval of 20 to 26 percent. With placebo plus docetaxel in the ITT population (n equals 625), ORR was 14 percent of patients with a 95 percent confidence interval of 11 to 17 percent. The P value was less than 0.001. CYRAMZA plus docetaxel achieved a 64 percent increase in ORR when compared to placebo plus docetaxel. ORR was defined as complete response plus partial response. ORR does not include stable disease. Disease progression and tumor response were assessed by investigators in accordance with Response Evaluation Criteria in Solid Tumors (RECIST) 1.1.
* ORR=complete response plus partial response; ORR does not include stable disease. Disease progression and tumor response were assessed by investigators in accordance with Response Evaluation Criteria in Solid Tumors (RECIST) 1.12
REVEL Trial Design (N=1253)
The phase III REVEL trial evaluated the efficacy and safety of CYRAMZA plus docetaxel vs placebo plus docetaxel in patients with locally advanced or metastatic NSCLC with disease progression on or after platinum-based chemotherapy. Major efficacy outcome measure was overall survival. Supportive efficacy outcome measures were progression-free survival and overall response rate. All patients were required to have Eastern Cooperative Oncology Group performance status 0 or 1. Patients were randomized 1:1 to receive either CYRAMZA 10 mg/kg (n=628) or placebo (n=625), in combination with docetaxel† at 75 mg/m2 every 21 days.
† 24 patients at East Asian sites received a starting dose of docetaxel at 60 mg/m2 every 3 weeks.
A 64% DCR was observed with CYRAMZA + docetaxel in the ITT population3
Supportive Outcome Measure: DCR - Percentage of Patients (95% CI)‡
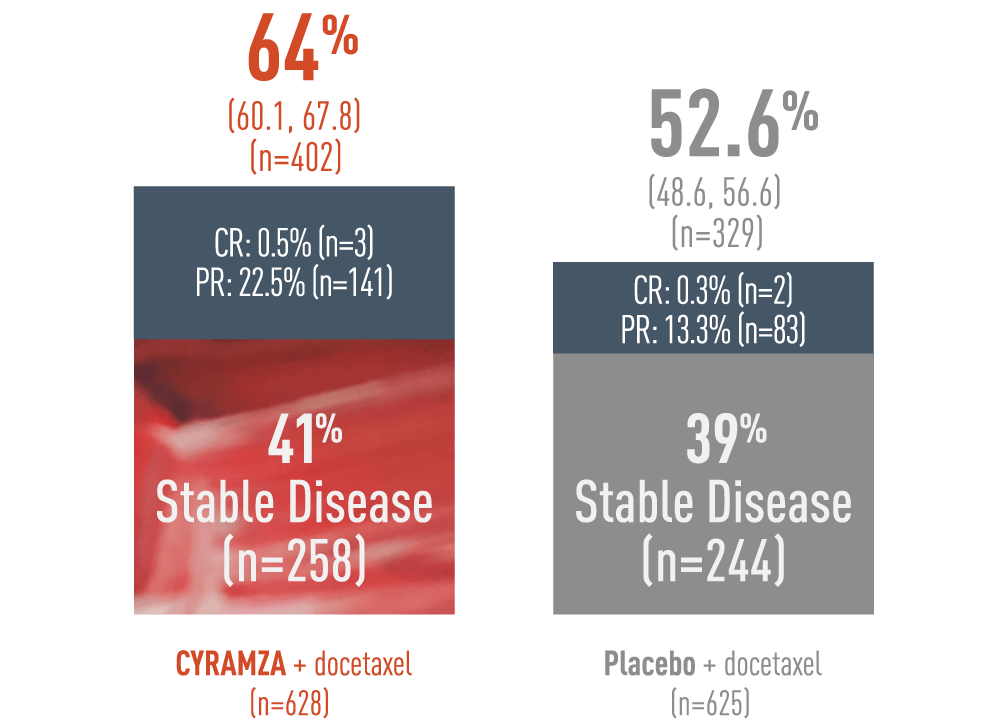
Disease control rate (DCR) was pre-specified secondary endpoint of the REVEL trial. DCR was defined as complete response (CR) plus partial response (PR) plus stable disease. The following DCR results of the REVEL trial are presented in a bar chart. With CYRAMZA plus docetaxel (n equals 402), the DCR was 64 percent of patients with a 95 percent confidence interval of 60.1 to 67.8 percent.In the CYRAMZA plus docetaxel arm, 0.5 percent of patients (n equals 3) achieved a CR, 22.5 percent of patients (n equals 141) experienced a PR, and 41 percent of patients (n equals 258) maintained stable disease. With placebo plus docetaxel (n equals 329), the DCR was 52.6 percent of patients with a 95 percent confidence interval of 48.6 to 56.6 percent. In the placebo plus docetaxel arm 0.3 percent of patients (n equals 2) achieved a CR, 13.3 percent of patients (n equals 83) experienced a PR, and 39 percent of patients (n equals 244) maintained stable disease. DCR was not controlled for type 1 error, and treatment differences observed cannot be regarded as statistically significant.
Disease Control Rate (DCR) was a pre-specified secondary endpoint. The DCR analysis was not controlled for type 1 error and therefore treatment differences cannot be regarded as statistically significant.
‡ Disease Control Rate was defined as complete response + partial response + stable disease
SELECT IMPORTANT SAFETY INFORMATION
Infusion-Related Reactions
- IRR, including severe and lifethreatening IRR, occurred in CYRAMZA clinical trials. Symptoms of IRR included rigors/tremors, back pain/spasms, chest pain and/or tightness, chills, flushing, dyspnea, wheezing, hypoxia, and paresthesia. In severe cases, symptoms included bronchospasm, supraventricular tachycardia, and hypotension. In 2137 patients with various cancers treated with CYRAMZA in which premedication was recommended or required, the incidence of all Grade IRR ranged from <1- 9%. Grade 3-5 IRR incidence was <1%.
- Premedicate prior to each CYRAMZA infusion. Monitor patients during the infusion for signs and symptoms of IRR in a setting with available resuscitation equipment. Reduce the infusion rate by 50% for Grade 1-2 IRR. Permanently discontinue CYRAMZA for Grade 3- 4 IRR.
ORR results for CYRAMZA were consistent between the ITT population and patients with rapidly progressing disease§ when added to docetaxel1,4
Post hoc exploratory subgroup analyses: Patients with rapid progression§ on initial platinum-based therapy (≤12 weeks; n=209)4
REVEL Subgroup ORR: Percentage of Patients (95% CI)4
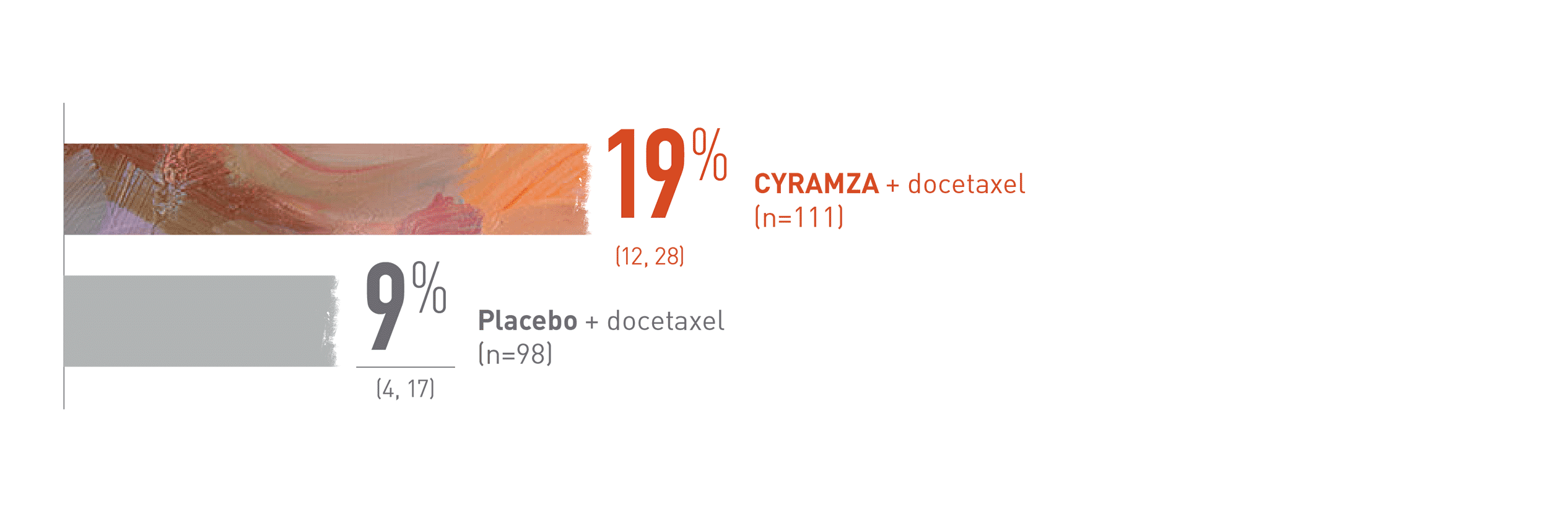
This is a post hoc exploratory subgroup analysis of the REVEL trial overall response rate (ORR) for patients with rapid disease progression on initial platinum-based therapy (n equals 209) which is presented in a bar chart.
With CYRAMZA plus docetaxel (n equals 111), ORR was 19 percent of patients with a 95 percent confidence interval of 12 to 28 percent. With placebo plus docetaxel (n equals 98), ORR was 9 percent of patients with a 95 percent confidence interval of 4 to 17 percent. The REVEL trial was not adequately powered or error-controlled for subgroup analysis. Treatment differences in this subgroup cannot be regarded as statistically significant.
Rapidly progressing disease is defined as time to progression within 12 weeks after starting initial platinum-based treatment. ORR equals complete response plus partial response; does not include stable disease. Disease progression and tumor response were assessed by investigators in accordance with Response Evaluation Criteria in Solid Tumors (RECIST).
REVEL EXPLORATORY ANALYSIS2,4,5:
The REVEL trial was not adequately powered or error-controlled for subgroup analysis. Treatment differences observed in this subgroup cannot be regarded as statistically significant. The analysis described here was post hoc and exploratory.
A 49.5% DCR was observed with CYRAMZA + docetaxel in the rapidly progressing‖ population6
DCR - Percentage of Patients (95% CI)6
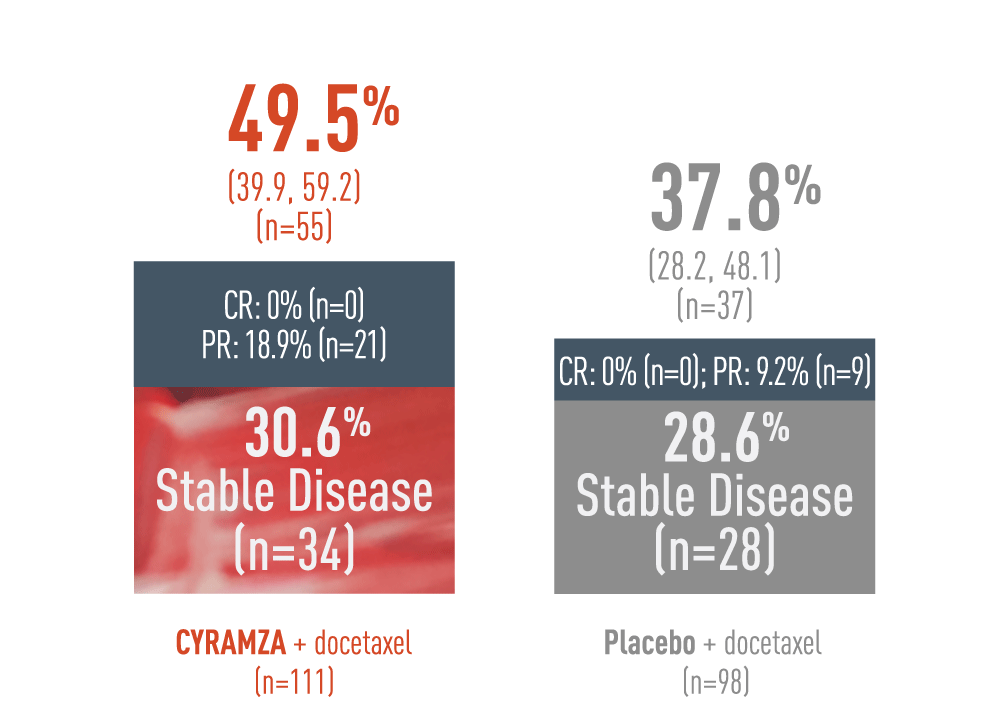
This is a post hoc exploratory subgroup analysis of the REVEL trial disease control rate (DCR) for patients with rapid disease progression on initial platinum-based therapy. The reults are presented in a bar chart.
With CYRAMZA plus docetaxel (n equals 111), the DCR was 49.5 percent of patients (n equals 55) with a 95 percent confidence interval of 39.9 to 59.2 percent. In the CYRAMZA plus docetaxel arm, 0 percent of patients (n equals 0) achieved a complete response (CR), 18.9 percent of patients (n equals 21) experienced a partial response (PR), and 30.6 percent of patients (n equals 34) maintained stable disease. With placebo plus docetaxel (n equals 98), the DCR was 37.8 percent of patients (n equals 37) with a 95 percent confidence interval of 28.2 to 48.1 percent. In the placebo plus docetaxel arm achieved 0 percent of patients (n equals 0) achieved a CR, 9.2 percent of patients (n equals 9) experienced a PR, and 28.6 percent of patients (n equals 28) maintained stable disease.
Rapidly progressing disease is defined as time to progression within 12 weeks after starting initial platinum-based treatment. The REVEL trial was not adequately powered or error-controlled for subgroup analyses. Treatment differences observed cannot be regarded as statistically significant. The analyses described here is post hoc and exploratory. DCR was defined as CR plus PR plus stable disease.
§‖ Rapidly progressing disease is defined as time to progression within 12 weeks after starting initial platinum-based treatment.
SELECT IMPORTANT SAFETY INFORMATION
Worsening of Preexisting Hepatic Impairment
- Clinical deterioration, manifested by new onset or worsening encephalopathy, ascites, or hepatorenal syndrome, was reported in patients with Child-Pugh B or C cirrhosis who received single agent CYRAMZA. Use CYRAMZA in patients with Child-Pugh B or C cirrhosis only if the potential benefits of treatment are judged to outweigh the risks of clinical deterioration.
- Based on safety data from REACH-2, in patients with Child-Pugh A liver cirrhosis, the pooled incidence of hepatic encephalopathy and hepatorenal syndrome was higher for patients who received CYRAMZA (6%) compared to patients who received placebo (0%).
Watch video: REVEL trial outcomes in patients with rapid progression
§ Rapidly progressing disease is defined as time to progression within 12 weeks after starting initial platinum-based treatment.4
CI=confidence interval; CR=complete response; DCR=disease control rate; ECOG=Eastern Cooperative Oncology Group; ITT=intent-to-treat; mNSCLC=metastatic non-small cell lung cancer; ORR=overall response rate; OS=overall survival; PFS=progression-free survival; PR=partial response; PS=performance status; RECIST=Response Evaluation Criteria in Solid Tumors; SD=stable disease.
References
- CYRAMZA (ramucirumab) package insert. Indianapolis, IN: Eli Lilly and Company; 2021.
- Garon EB, Ciuleanu T-E, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665-673.
- Supplement to: Garon EB, Ciuleanu T-E, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665-673.
- Reck M, Shepherd F, Pérol M, et al. Effects of second-line ramucirumab after rapid time to progression on first-line therapy: subgroup analysis of REVEL in advanced non-small cell lung cancer. Oral presentation presented at: IASLC 18th World Conference on Lung Cancer; October 15-18, 2017; Yokohama, Japan.
- Data on File, Lilly USA, LLC, DOF-RB-US-0006.
- Data on File, Lilly USA, LLC, DOF-RB-US-0044.