
CYRAMZA + erlotinib helped patients with EGFR mut+ mNSCLC reach more than 19 months median PFS1
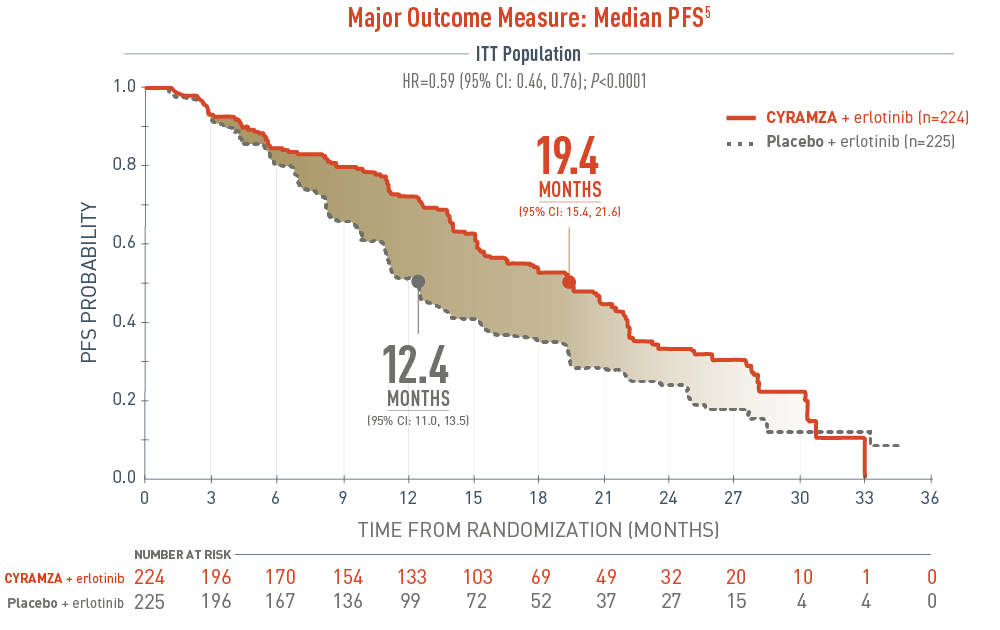
Progression-free survival (PFS) was a major outcome measure of the RELAY trial. The following PFS results for epidermal growth factor receptor gene mutant (EGFR mut+) metastatic non-small cell lung cancer (mNSCLC) patients are presented in a Kaplan-Meier curve. With CYRAMZA plus erlotinib in the intent-to-treat (ITT) population (n equals 224), median PFS was 19.4 months with a 95 percent confidence interval of 15.4 to 21.6 months. With placebo plus erlotinib in the ITT population (n equals 225), median PFS was 12.4 months with a 95 percent confidence interval of 11.0 to 13.5 months. The hazard ratio was 0.59 with a 95 percent confidence interval of 0.46 to 0.76 and a P value less than 0.0001.
- The percentage of events at the time of analysis was 55% (122 patients) and 70% (158 patients) in the CYRAMZA + erlotinib and placebo + erlotinib treatment arms, respectively1
- 4 of 122 events in CYRAMZA-treated patients and 1 of 158 events in placebo-treated patients were deaths1
- The major efficacy outcome measure was PFS as assessed by the investigator RECIST v1.11
28.1% of patients treated with CYRAMZA + erlotinib and 49.3% of those treated with placebo + erlotinib had their disease progress at 1 year2
The RELAY trial evaluated the efficacy and safety of CYRAMZA + erlotinib vs placebo + erlotinib in previously untreated patients with EGFR mut+ mNSCLC. Major outcome measure was PFS. Supportive outcome measures included OS, ORR, and DoR. Patients were stratified by EGFR mutation status (exon 21 vs exon 19), geographic region (East Asia vs other), gender, and local EGFR testing method (therascreen® and cobas® vs other PCR and sequencing-based methods). All patients were required to have ECOG PS 0 or 1. Patients were randomized 1:1 to receive CYRAMZA 10 mg/kg (n=224) or placebo (n=225) every 2 weeks + erlotinib 150 mg/day.1,3
ORR=CR+PR; ORR does not include SD.3.
For patients on CYRAMZA + erlotinib who had a response, the median DoR was 18.0 months with CYRAMZA + erlotinib vs 11.1 months with placebo + erlotinib1,3
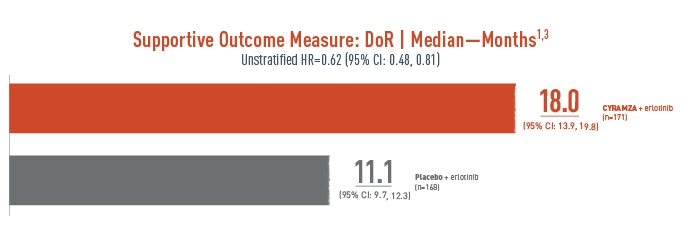
Duration of a response (DoR) was a supportive outcome measure of the RELAY trial. The following results are presented.
With CYRAMZA plus erlotinib (n equals 171), median DoR was 18.0 months with a 95 percent confidence interval of 13.9 to 19.8 months. With placebo plus erlotinib (n equals 168), median DoR was 11.1 months with a 95 percent confidence interval of 9.7 to 12.3 months. The unstratified hazard ratio was 0.62 with a 95 percent confidence interval of 0.48 to 0.81.
- The percentage of events at the time of analysis was 59% (101 patients) and 76% (128 patients) in the CYRAMZA + erlotinib and placebo + erlotinib treatment arms, respectively3
DoR was a prespecified secondary efficacy endpoint. DoR was not powered or controlled for type 1 error, and treatment differences observed cannot be regarded as statistically significant.
SELECT IMPORTANT SAFETY INFORMATION
Arterial Thromboembolic Events (ATEs)
- Serious, sometimes fatal, ATEs, including myocardial infarction, cardiac arrest, cerebrovascular accident, and cerebral ischemia, occurred across clinical trials. In 2137 patients with various cancers treated with CYRAMZA, the incidence of all Grade ATE was 1-3%. Grade 3-5 ATE incidence was <1-2%.
- Permanently discontinue CYRAMZA in patients who experience an ATE.
Supportive Outcome Measure: Interim OS1
HR=0.83 [95% CI: 0.53, 1.30]
CYRAMZA + erlotinib [n=224] vs Placebo + erlotinib [n=225]
- The interim OS data at the time of data cutoff for the primary analysis were immature1,3
- At the time of the final analysis of PFS, OS data were not mature as only 26% of planned events for the final analysis had occurred1
-
A final OS analysis is planned when ≥300 events have occurred3
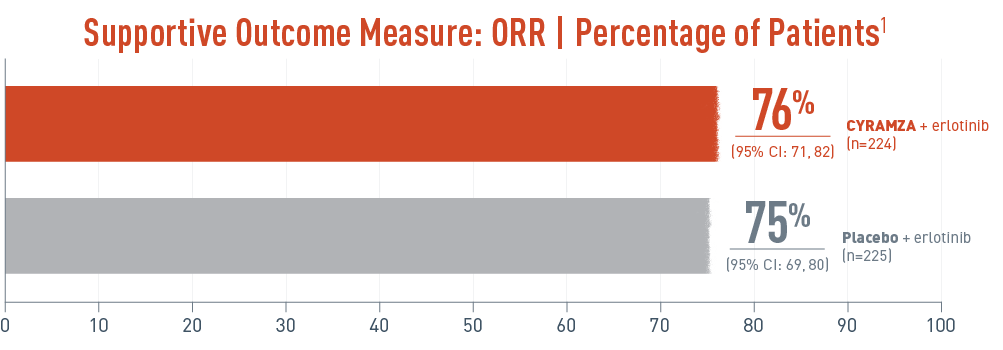
Overall response rate (ORR) was a supportive outcome measure of the RELAY trial. The following ORR results for epidermal growth factor receptor gene mutant (EGFR mut+) metastatic non-small cell lung cancer (mNSCLC) patients are presented. With CYRAMZA plus erlotinib in the intent-to-treat (ITT) population (n equals 224), ORR was 76 percent of patients with a 95 percent confidence interval of 71 to 82 percent. With placebo plus erlotinib in the ITT population (n equals 225), ORR was 75 percent with a 95 percent confidence interval of 69 to 80 percent. ORR was a prespecified secondary efficacy endpoint. ORR was not powered or controlled for type 1 error, and treatment differences observed cannot be regarded as statistically significant.
- ORR was defined as CR + PR. ORR does not include stable disease3
- Disease progression and tumor response were assessed by investigators in accordance with RECIST v1.13
ORR was a prespecified secondary efficacy endpoint. ORR was not powered or controlled for type 1 error, and treatment differences observed cannot be regarded as statistically significant.
Disease Control (DC)* was 95% in the CYRAMZA + erlotinib arm and 96% in the placebo + erlotinib arm.3
Prespecified Supportive Outcome Measure: DC3*
CYRAMZA + erlotinib (n=213/224)
95% (95% CI: 92, 98)
CR=1% (n=3)
PR=75% (n=168)
SD=19% (n=42)
Placebo + erlotinib (n=215/225)
96% (95% CI: 93, 98)
CR=1% (n=2)
PR=74% (n=166)
SD=21% (n=47)
* DC was defined as CR + PR + SD.
DC was a prespecified secondary efficacy endpoint. This endpoint was not powered or controlled for type 1 error, and treatment differences observed cannot be regarded as statistically significant.
SELECT IMPORTANT SAFETY INFORMATION
Hypertension
- An increased incidence of severe hypertension occurred in patients receiving CYRAMZA. Across five clinical studies, excluding RELAY, in 1916 patients with various cancers treated with CYRAMZA, the incidence of all Grade hypertension ranged from 11-26%. Grade 3-5 hypertension incidence ranged from 6-15%. In 221 patients with NSCLC receiving CYRAMZA in combination with erlotinib in the RELAY study, the incidence of new or worsening hypertension was higher (45%), as was the incidence of Grade 3-5 hypertension (24%). Of the patients experiencing new or worsening hypertension in RELAY (N=100 CYRAMZA and erlotinib; N=27 placebo and erlotinib), 13% of those treated with CYRAMZA and erlotinib required initiation of 3 or more antihypertensive medications compared to 4% of patients treated with placebo and erlotinib.
-
Control hypertension prior to initiating treatment with CYRAMZA. Monitor blood pressure every two weeks or more frequently as indicated during treatment. Withhold CYRAMZA for severe hypertension until medically controlled. Permanently discontinue CYRAMZA for medically significant hypertension that cannot be controlled with antihypertensive therapy or in patients with hypertensive crisis or hypertensive encephalopathy.
In RELAY, median PFS was consistent across the ITT and subgroup populations of exon 21 (L858R) substitution and exon 19 deletion3
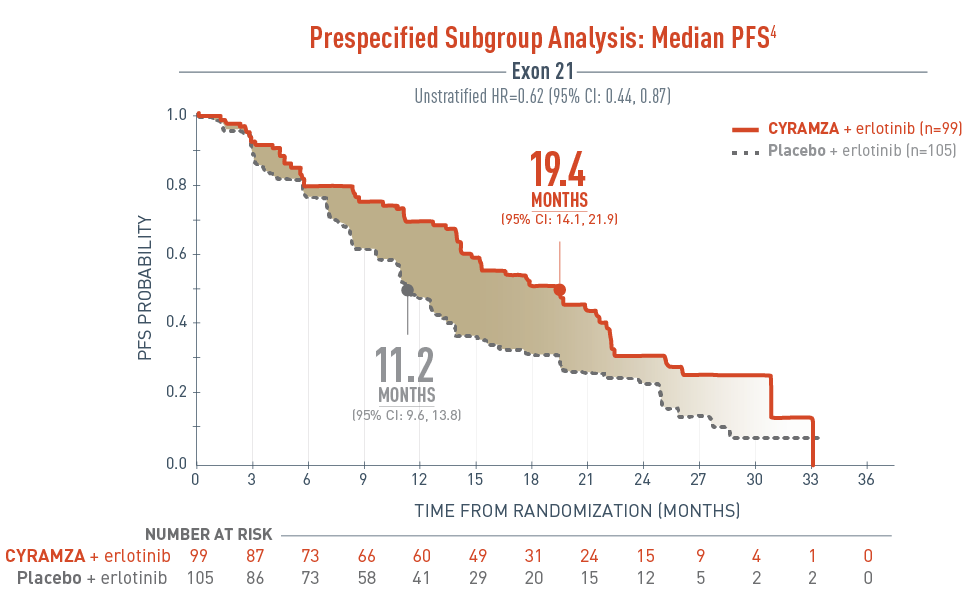
The RELAY trial included a prespecified subgroup analysis assessing the progression-free survival (PFS) in patients with exon 21 substitution.
The results were as follows, CYRAMZA plus erlotinib (n equals 99), median PFS was 19.4 months with a 95 percent confidence interval of 14.1 to 21.9 months, and placebo plus erlotinib (n equals 105), median PFS was 11.2 months with a 95 percent confidence interval of 9.6 to 13.8 months. The unstratified hazard ratio was 0.62 with a 95 percent confidence interval of 0.44 to 0.87.
- The percentage of events at the time of analysis was 58.6% (58 patients) and 70.5% (74 patients) in the CYRAMZA + erlotinib and placebo + erlotinib treatment arms, respectively3
-
2 of 58 events in CYRAMZA-treated patients and 1 of 74 events in the placebo-treated patients were deaths4
The RELAY trial was not adequately powered or error controlled for subgroup analysis. Treatment differences observed in this subgroup cannot be regarded as statistically significant.
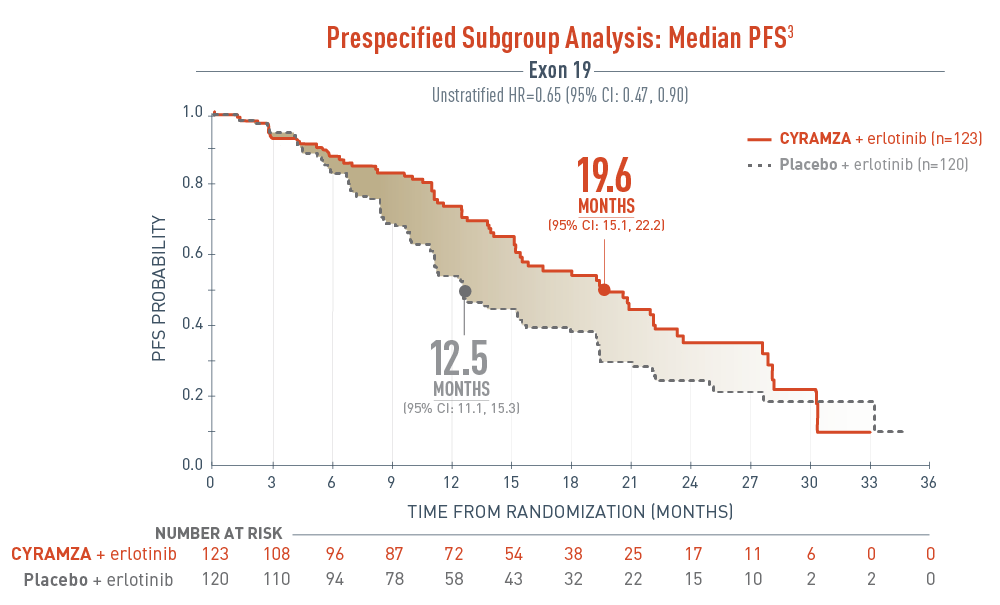
The RELAY trial included a prespecified subgroup analysis assessing the progression-free survival (PFS) in patients with exon 19 deletion.
The results were as follows, CYRAMZA plus erlotinib (n equals 123), median PFS was 19.6 months with a 95 percent confidence interval of 15.1 to 22.2 months, and placebo plus erlotinib (n equals 120), median PFS was 12.5 months with a 95 percent confidence interval of 11.1 to 15.3 months. The unstratified hazard ratio was 0.65 with a 95 percent confidence interval of 0.47 to 0.90.
- The percentage of events at the time of analysis was 52.0% (64 patients) and 70.0% (84 patients) in the CYRAMZA + erlotinib and placebo + erlotinib treatment arms, respectively3
- 2 of 64 events in CYRAMZA-treated patients and 0 of 84 events in the placebo-treated patients were deaths4
The RELAY trial was not adequately powered or error controlled for subgroup analysis. Treatment differences observed in this subgroup cannot be regarded as statistically significant.
SELECT IMPORTANT SAFETY INFORMATION
Infusion-Related Reactions (IRR)
- IRR, including severe and life-threatening IRR, occurred in CYRAMZA clinical trials. Symptoms of IRR included rigors/tremors, back pain/spasms, chest pain and/or tightness, chills, flushing, dyspnea, wheezing, hypoxia, and paresthesia. In severe cases, symptoms included bronchospasm, supraventricular tachycardia, and hypotension. In 2137 patients with various cancers treated with CYRAMZA in which premedication was recommended or required, the incidence of all Grade IRR ranged from <1- 9%. Grade 3-5 IRR incidence was <1%.
- Premedicate prior to each CYRAMZA infusion. Monitor patients during the infusion for signs and symptoms of IRR in a setting with available resuscitation equipment. Reduce the infusion rate by 50% for Grade 1-2 IRR. Permanently discontinue CYRAMZA for Grade 3- 4 IRR.
Prespecified subgroup analyses of PFS5
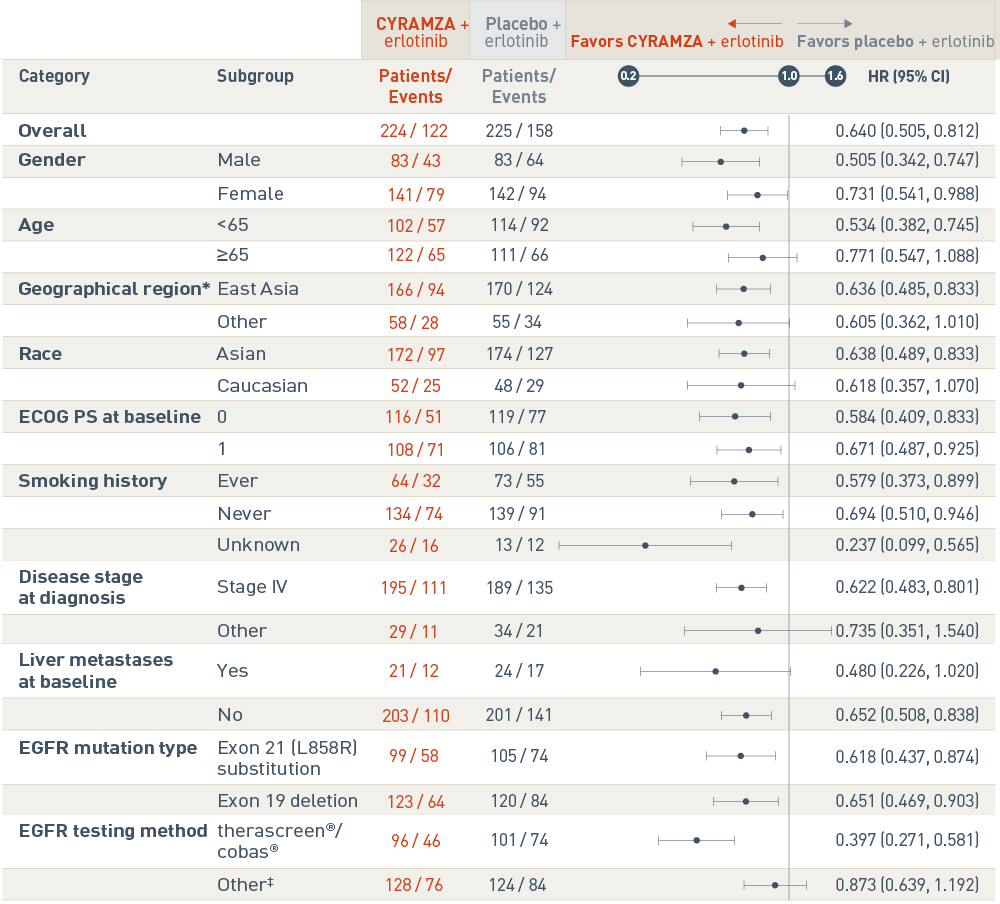
The RELAY trial included several prespecified patient subgroup analyses of progression-free survival (PFS). The following results are presented in a Forest plot.
Overall, events were reported in 122 of 224 patients in the CYRAMZA plus erlotinib arm, and in 158 of 225 patients in the placebo plus erlotinib arm. The CYRAMZA plus erlotinib arm was favored. The hazard ratio was 0.640 with a 95 percent confidence interval 0.505 to 0.812.
In the male subgroup, events were reported in 43 of 83 patients in the CYRAMZA plus erlotinib arm, and in 64 of 83 patients in the placebo plus erlotinib arm. The CYRAMZA plus erlotinib group was favored. The hazard ratio was 0.505 with a 95 percent confidence interval 0.342 to 0.747. In the female subgroup, events were reported in 79 of 141 patients in the CYRAMZA plus erlotinib arm, and in 94 of 142 patients in the placebo plus erlotinib arm. The CYRAMZA plus erlotinib group was favored. The hazard ratio was 0.731 with a 95 percent confidence interval 0.541 to 0.988.
In the subgroup of patients who were less than 65 years of age, events were reported in 57 of 102 patients in the CYRAMZA plus erlotinib arm, and in 92 of 114 patients in the placebo plus erlotinib arm. The CYRAMZA plus erlotinib group was favored. The hazard ratio was 0.534 with a 95 percent confidence interval 0.382 to 0.745. In the subgroup of patients who were 65 years of age or older, events were reported in 65 of 122 patients in the CYRAMZA plus erlotinib arm, and in 66 of 111 patients in the placebo plus erlotinib arm. The hazard ratio was 0.771 with a 95 percent confidence interval 0.547 to 1.088.
In the subgroup of patients from East Asia, events were reported in 94 of 166 patients in the CYRAMZA plus erlotinib arm, and in 124 of 170 patients in the placebo plus erlotinib arm. The CYRAMZA plus erlotinib group was favored. The hazard ratio was 0.636 with a 95 percent confidence interval 0.485 to 0.833 In the subgroup of patients from Other regions, events were reported in 28 of 58 patients in the CYRAMZA plus erlotinib arm, and in 34 of 55 patients in the placebo plus erlotinib arm. The hazard ratio was 0.605 with a 95 percent confidence interval 0.362 to 1.010. East Asia includes South Korea, Hong Kong, Japan, and Taiwan, and Other includes Canada, France, Germany, Italy, Romania, Spain, Turkey, the United States, and the United Kingdom.
In the Asian subgroup, events were reported in 97 of 172 patients in the CYRAMZA plus erlotinib arm, and in 127 of 174 patients in the placebo plus erlotinib arm. The CYRAMZA plus erlotinib group was favored. The hazard ratio was 0.638 with a 95 percent confidence interval 0.489 to 0.833. In the Caucasian subgroup, events were reported in 25 of 52 patients in the CYRAMZA plus erlotinib arm, and in 29 of 48 patients in the placebo plus erlotinib arm. The hazard ratio was 0.618 with a 95 percent confidence interval 0.357 to 1.070.
In the subgroup of patients with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0, events were reported in 51 of 116 patients in the CYRAMZA plus erlotinib arm, and in 77 of 119 patients in the placebo plus erlotinib arm. The CYRAMZA plus erlotinib group was favored. The hazard ratio was 0.584 with a 95 percent confidence interval 0.409 to 0.833. In the subgroup of patients with an ECOG PS of 1, events were reported in 71 of 108 patients in the CYRAMZA plus erlotinib arm, and in 81 of 106 patients in the placebo plus erlotinib arm. The CYRAMZA plus erlotinib group was favored. The hazard ratio was 0.671 with a 95 percent confidence interval 0.487 to 0.925.
In the subgroup of patients who reported as ever having smoked, events were reported in 32 of 64 patients in the CYRAMZA plus erlotinib arm, and in 55 of 73 patients in the placebo plus erlotinib arm. The CYRAMZA plus erlotinib group was favored. The hazard ratio was 0.579 with a 95 percent confidence interval 0.373 to 0.899. In the subgroup of patients who reported as never having smoked, events were reported in 74 of 134 patients in the CYRAMZA plus erlotinib arm, and in 91 of 139 patients in the placebo plus erlotinib arm. The CYRAMZA plus erlotinib group was favored. The hazard ratio was 0.694 with a 95 percent confidence interval 0.510 to 0.946. In the subgroup of patients whose smoking history was unknown, events were reported in 16 of 26 patients in the CYRAMZA plus erlotinib arm, and in 12 of 13 patients in the placebo plus erlotinib arm. The CYRAMZA plus erlotinib group was favored. The hazard ratio was 0.237 with a 95 percent confidence interval 0.099 to 0.565.
In the subgroup with stage IV disease at diagnosis, events were reported in 111 of 195 patients in the CYRAMZA plus erlotinib arm in 135 of 189 patients, and in the placebo plus erlotinib arm. The CYRAMZA plus erlotinib group was favored. The hazard ratio was 0.622 with a 95 percent confidence interval 0.483 to 0.801. In the subgroup with other than stage IV disease at diagnosis, events were reported in 11 of 29 patients in the CYRAMZA plus erlotinib arm, and in 21 of 34 patients in the placebo plus erlotinib arm. The hazard ratio was 0.735 with a 95 percent confidence interval 0.351 to 1.540.
In the subgroup with liver metastases at baseline, events were reported in 12 of 21 patients in the CYRAMZA plus erlotinib arm, and in 17 of 24 patients in the placebo plus erlotinib arm. The hazard ratio was 0.480 with a 95 percent confidence interval 0.226 to 1.020. In the subgroup without liver metastases at baseline, events were reported in 110 of 203 patients in the CYRAMZA plus erlotinib arm, and in 141 of 201 patients in the placebo plus erlotinib arm. The CYRAMZA plus erlotinib group was favored. The hazard ratio was 0.652 with a 95 percent confidence interval 0.508 to 0.838.
In the subgroup with Exon 21 (L858R) substitution, events were reported in 58 of 99 patients in the CYRAMZA plus erlotinib arm, and in 74 of 105 patients in the placebo plus erlotinib arm. The CYRAMZA plus erlotinib group was favored. The hazard ratio was 0.618 with a 95 percent confidence interval 0.437 to 0.874. In the subgroup with Exon 19 deletion, events were reported in 64 of 123 patients in the CYRAMZA plus erlotinib arm, and in 84 of 120 patients in the placebo plus erlotinib arm. The CYRAMZA plus erlotinib group was favored. The hazard ratio was 0.651 with a 95 percent confidence interval 0.469 to 0.903.
In the subgroup whose epidermal growth factor receptor (EGFR) mutation status was determined by therascreen®/cobas®, events were reported in 46 of 96 patients in the CYRAMZA plus erlotinib arm, and in 74 of 101 patients in the placebo plus erlotinib arm. The CYRAMZA plus erlotinib group was favored. The hazard ratio was 0.397 with a 95 percent confidence interval 0.271 to 0.581 In the subgroup whose EGFR mutation status was determined by a method other than therascreen®/cobas®, events were reported in 76 of 128 patients in the CYRAMZA plus erlotinib arm, and in 84 of 124 patients in the placebo plus erlotinib arm. The hazard ratio was 0.837 with a 95 percent confidence interval 0.639 to 1.192. The testing method for 1 patient was missing on the case report form (CRF). Patient was stratified by other PCR and sequencing-based method in interactive web-response system (IWRS). Other trademarks are the property of their respective owners.
† East Asia includes South Korea, Hong Kong, Japan, and Taiwan, and Other includes Canada, France, Germany, Italy, Romania, Spain, Turkey, the United States, and the United Kingdom.
‡ Testing method for 1 patient was missing on the CRF. Patient was stratified by other PCR and sequencing-based method in IWRS.
Other trademarks are the property of their respective owners.
These analyses were not adjusted for multiplicity or powered to detect the effect of CYRAMZA + erlotinib within subgroups. Therefore, no conclusions of statistical significance can be drawn.
Consider CYRAMZA + erlotinib for your patients with EGFR mut+ mNSCLC with an exon 21 (L858R) substitution or exon 19 deletion
Ask your sales representative about RELAY
CI=confidence interval; CR=complete response; CRF=case report form; DoR=duration of response; ECOG=Eastern Cooperative Oncology Group; EGFR=epidermal growth factor receptor; HR=hazard ratio; ITT=intent-to-treat; IWRS=interactive web-response system; mNSCLC=metastatic non-small cell lung cancer; mut+=mutation-positive; ORR=overall response rate; OS=overall survival; PCR=polymerase chain reaction; PFS=progression-free survival; PR=partial response; PS=performance status; RECIST=Response Evaluation Criteria in Solid Tumors; SD=stable disease.
References
- CYRAMZA (ramucirumab) package insert. Indianapolis, IN: Eli Lilly and Company; 2021.
- Data on File, Lilly USA, LLC, DOF-RB-US-0069.
- Nakagawa K, Garon EB, Seto T, et al; for RELAY Study Investigators. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial [published online October 4, 2019]. Lancet Oncol. doi:10.1016/S1470-2045(19)30634-5.
- Data on File, Lilly USA, LLC, DOF-RB-US-0074.
- Data on File, Lilly USA, LLC, DOF-RB-US-0071.