
CYRAMZA® (ramucirumab) + erlotinib is the first and only FDA-approved 1L combination therapy for patients with EGFR mut+ mNSCLC1
RELAY: A global, multicenter, randomized, double-blind, placebo-controlled phase III trial of CYRAMZA + erlotinib vs placebo + erlotinib in previously untreated patients with EGFR mut+ mNSCLC1,2
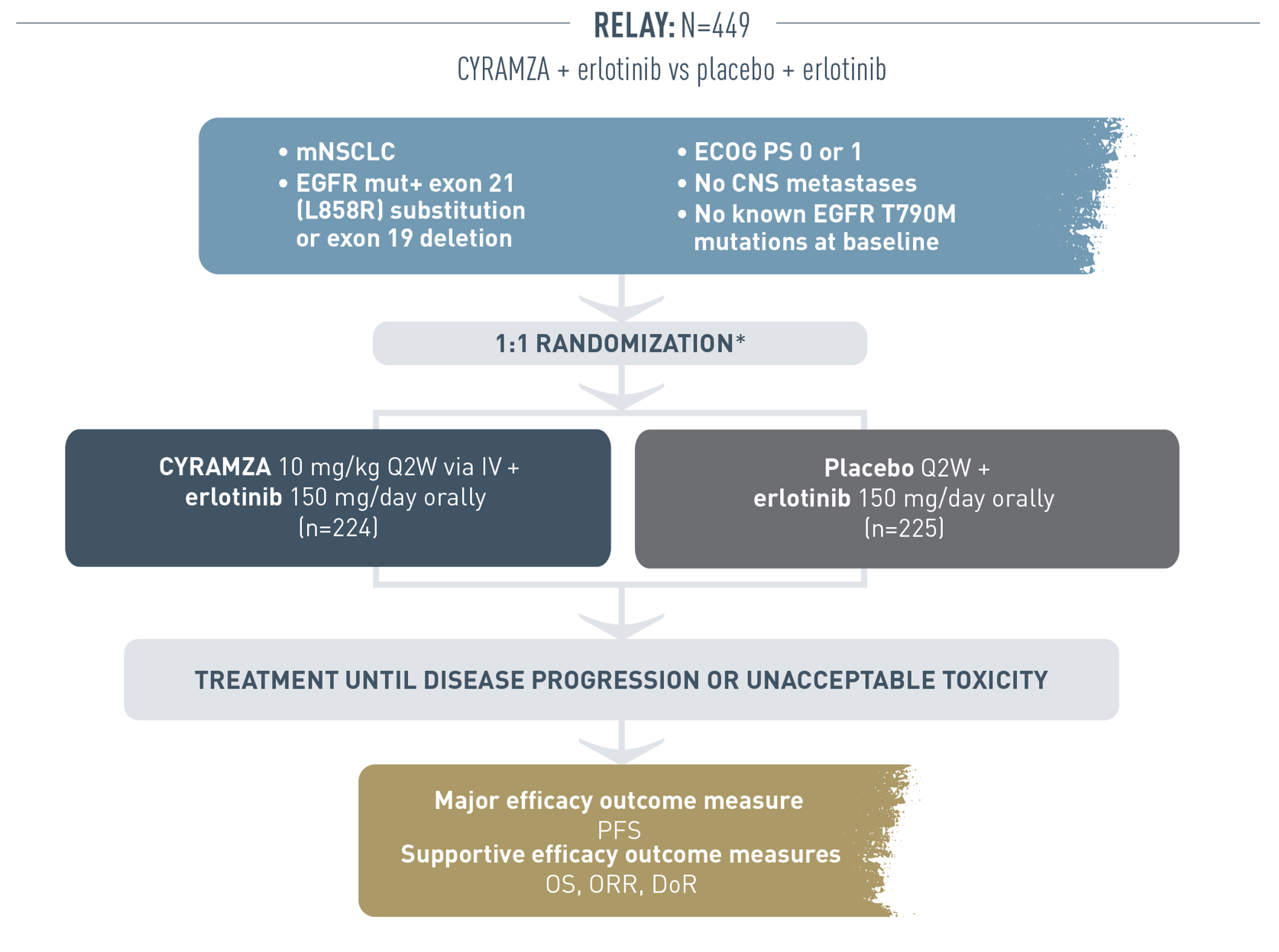
The following clinical study design is for the RELAY trial.
This global, multicenter, randomized, double-blind trial, placebo-controlled phase III trial was conducted in previously untreated patients with non-small cell lung cancer (NSCLC) who were positive for a mutation in epidermal growth factor receptor gene (EGFR mut+). Patients were included in the trial if they had an exon 21 (L858R) substitution or exon 19 deletion, an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, no CNS metastases, and no known EGFR T790M mutations at baseline. Randomization was stratified by geographic region (East Asia versus other), gender, EGFR mutation (exon 21 substitution versus exon 19 deletion), and local EGFR testing method (therascreen® and cobas® versus other PCR and sequencing-based methods). Patients (N equals 449) were randomized (1 to 1). In one arm (n equals 224), patients received CYRAMZA 10 milligrams per kilogram once every 2 weeks by intravenous administration plus erlotinib 150 milligrams per day orally. In the other arm (n equals 225), patients received placebo once every 2 weeks plus erlotinib 150 milligrams per day orally. Treatment was administered until disease progression or unacceptable toxicity. The major efficacy outcome measure was progression-free survival (PFS) and the supportive efficacy outcome measures were overall survival (OS), overall response rate (ORR), and duration of response (DoR). ORR was defined as complete response (CR) plus partial response (PR). ORR did not include stable disease (SD).
* Stratification factors: Geographic region (East Asia vs other), gender, EGFR mutation (exon 21 substitution vs exon 19 deletion mutation), and local EGFR testing method (therascreen® and cobas® vs other PCR and sequencing-based methods).
ORR= CR + PR; ORR does not include SD.2
Prespecified analysis included, but was not limited to, evaluation of PFS outcomes by exon 21 and exon 19 mutations.2
SELECT IMPORTANT SAFETY INFORMATION
Impaired Wound Healing
- CYRAMZA has the potential to adversely affect wound healing. CYRAMZA has not been studied in patients with serious or non-healing wounds.
- Withhold CYRAMZA for 28 days prior to elective surgery. Do not administer CYRAMZA for at least 2 weeks following a major surgical procedure and until adequate wound healing. The safety of resumption of CYRAMZA after resolution of wound healing complications has not been established.
Patient demographics and clinical characteristics of patients at baseline (ITT population)2
Parameter
|
CYRAMZA + erlotinib (n=224)
|
Placebo + erlotinib (n=225)
| |
|---|---|---|---|
| Age | Median (IQR), years ≥65 years | CYRAMZA + erlotinib (n=224): 65 (57-71) 122 (54%) | Placebo + erlotinib (n=225): 64 (56-70) 111 (49%) |
| Sex | Female Male | CYRAMZA + erlotinib (n=224): 141 (63%) 83 (37%) | Placebo + erlotinib (n=225): 142 (63%) 83 (37%) |
| Race† | Asian White Other | CYRAMZA + erlotinib (n=224): 172 (77%) 52 (23%) 0 | Placebo + erlotinib (n=225): 174 (77%) 48 (21%) 3 (1%) |
| Smoking status | Ever Never Unknown or missing | CYRAMZA + erlotinib (n=224): 64 (29%) 134 (60%) 26 (12%) | Placebo + erlotinib (n=225): 73 (32%) 139 (62%) 13 (6%) |
| Geographical region‡ | East Asia Other | CYRAMZA + erlotinib (n=224): 166 (74%) 58 (26%) | Placebo + erlotinib (n=225): 170 (76%) 55 (24%) |
| ECOG PS | 0 1 | CYRAMZA + erlotinib (n=224): 116 (52%) 108 (48%) | Placebo + erlotinib (n=225): 119 (53%) 106 (47%) |
| Pathological diagnosis at study entry | Adenocarcinoma NSCLC not otherwise specified | CYRAMZA + erlotinib (n=224): 215 (96%) 9 (4%) | Placebo + erlotinib (n=225): 218 (97%) 7 (3%) |
| Disease stage at diagnosis§ | Stage IV Other | CYRAMZA + erlotinib (n=224): 195 (87%) 29 (13%) | Placebo + erlotinib (n=225): 189 (84%) 36 (16%) |
| EGFR mutation type at randomization (eCRF) | Exon 19 deletion Exon 21 (L858R) substitution Missing Other | CYRAMZA + erlotinib (n=224): 123 (55%) 99 (44%) 1 (<1%)1 (<1%) | Placebo + erlotinib (n=225): 120 (53%) 105 (47%) 0 0 |
| EGFR testing method | therascreen® or cobas® Other PCR and sequencing-based methods | CYRAMZA + erlotinib (n=224): 96 (43%) 127 (57%) | Placebo + erlotinib (n=225): 101 (45%) 124 (55%) |
The following patient demographic and clinical characteristics at baseline for the intent-to-treat (ITT) population are for the RELAY trial.
For patients in the CYRAMZA plus erlotinib treatment arm (n equals 224), median (interquartile range; IQR) age was 65 years (range equals 57 to 71 years), 122 patients (54 percent) age 65 years or older, 141 patients (63 percent) female, 83 patients (37 percent) were male, 172 patients (77 percent) were Asian, 52 patients (23 percent) were White, 0 patients were classified as other for race, 64 patients (29 percent) had smoked at least once in their lifetime, 134 patients (60 percent) never smoked, 26 patients (12 percent) had an unknown or missing smoking status, 166 patients (74 percent) were from East Asia, 58 (26 percent) were from some other geographical region, 116 patients (52 percent) had an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0, and 108 patients (48 percent) had an ECOG PS score of 1. Of the patients in the CYRAMZA plus erlotinib treatment arm (n equals 224), 215 patients (96 percent) had adenocarcinoma at study entry, 9 patients (4 percent) had non-small cell carcinoma (NSCLC) not otherwise specified at study entry, 195 patients (87 percent) were stage IV at diagnosis, 29 patients (13 percent) were some other stage at diagnosis, 123 patients (55 percent) had exon 19 deletion as their epidermal growth factor receptor (EGFR) mutation type at randomization, 99 patients (44 percent) had exon 21 (L858R) substitution as their EGFR mutation type at randomization, 1 patient (less than 1 percent) had missing EGFR mutation information at randomization and 1 patient (less than 1 percent) had some other mutation. Ninety-six patients (43 percent) had the therascreen® or cobas® EGFR testing method and 127 patients (57 percent) had some other PCR and sequencing-based methods.
For patients in the placebo plus erlotinib treatment arm (n equals 225), median (IQR) age was 64 years (range equals 56 to 70 years), 111 patients (49 percent) age 65 years or older, 142 patients (63 percent) female, 83 patients (37 percent) were male, 174 patients (77 percent) were Asian, 48 patients (21 percent) were White, 3 patients (1 percent) were classified as Other for race, 73 patients (32 percent) had smoked at least once in their lifetime, 139 patients (62 percent) never smoked, 13 patients (6 percent) had an unknown or missing smoking status, 170 patients (76 percent) were from East Asia, 55 (24 percent) were from some other geographical region, 119 patients (53 percent) had an ECOG PS score of 0, and 106 patients (47 percent) had an ECOG PS score of 1. Of the patients in the CYRAMZA plus erlotinib treatment arm (n equals 224), 218 patients (97 percent) had adenocarcinoma at study entry, 7 patients (3 percent) had NSCLC not otherwise specified at study entry, 189 patients (84 percent) were stage IV at diagnosis, 36 patients (16 percent) were some other stage at diagnosis, 120 patients (53 percent) had exon 19 deletion as their EGFR mutation type at randomization, 105 patients (47 percent) had exon 21 (L858R) substitution as their EGFR mutation type at randomization, no patients had missing EGFR mutation information at randomization and no patients had other mutations. One hundred and one patients (45 percent) had the therascreen® or cobas® EGFR testing method and 124 patients (55 percent) had some other PCR and sequencing-based methods.
For race, "other" included American Indian or Alaska Native, black or African-American, or missing; data were missing for 1 patient in the placebo plus erlotinib group. For geographical regions, East Asia includes South Korea, Hong Kong, Japan, and Taiwan; other includes Canada, France, Germany, Italy, Romania, Spain, Turkey, the United States, and the United Kingdom.
All patients were required to have stage IV NSCLC at study entry. Patients with recurrent metastatic disease were permitted as long as the adjuvant or neoadjuvant therapy was completed at least 12 months prior to development of metastatic disease; previous adjuvant or neoadjuvant therapy was not required. At study entry, all patients (as per inclusion criteria) had metastatic stage IV disease (195 [87 percent] of 224 in the CYRAMZA plus erlotinib group versus 191 [85 percent] of 225 in the placebo group) or recurrent metastatic stage IV disease (29 [13 percent] versus 34 [15 percent]).
† Other included American Indian or Alaska Native, black or African-American, or missing; data were missing for 1 patient in the placebo + erlotinib group.
‡ East Asia includes South Korea, Hong Kong, Japan, and Taiwan; other includes Canada, France, Germany, Italy, Romania, Spain, Turkey, the United States, and the United Kingdom.
§ All patients were required to have stage IV NSCLC at study entry; patients with recurrent metastatic disease were permitted as long as the adjuvant or neoadjuvant therapy was completed at least 12 months prior to development of metastatic disease; previous adjuvant or neoadjuvant therapy was not required; at study entry, all patients (as per inclusion criteria) had metastatic stage IV disease (195 [87%] of 224 in the CYRAMZA + erlotinib group vs 191 [85%] of 225 in the placebo + erlotinib group) or recurrent metastatic stage IV disease (29 [13%] vs 34 [15%]).
Ask your sales representative about RELAY
1L=first-line; CNS=central nervous system; DoR=duration of response; ECOG=Eastern Cooperative Oncology Group; eCRF=electronic case report form; EGFR=epidermal growth factor receptor; IQR=interquartile range; ITT=intent-to-treat; IV=intravenous; mNSCLC=metastatic non-small cell lung cancer; mut+=mutation-positive; NSCLC=non-small cell lung cancer; ORR=overall response rate; OS=overall survival; PCR=polymerase chain reaction; PFS=progression-free survival; PS=performance status; SD=stable disease; Q2W=every 2 weeks.
References
- CYRAMZA (ramucirumab) package insert. Indianapolis, IN: Eli Lilly and Company; 2021.
- Nakagawa K, Garon EB, Seto T, et al; for RELAY Study Investigators. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial [published online October 4, 2019]. Lancet Oncol. doi:10.1016/S1470-2045(19)30634-5.