Patients with disease progression on or after platinum-based therapy have a high unmet need for options that can extend survival*1
Not all patients with mNSCLC progress at the same rate.1
There are some patients who know their situation but are not surrendering to it.
- Rapidly progressing disease† (≤12 weeks) on platinum-based treatment1
- ECOG PS 0 or 12
- Non-squamous or squamous histology2
- Disease progression on platinum-based therapy (ie, 20% growth as defined per RECIST criteria)2,3
- Increase in number of metastatic lesions2,3
- No contraindications for antiangiogenic therapy2
Can you picture one of your patients?
* Hypothetical patient example.
† Rapidly progressing disease is defined as time to progression within 12 weeks after starting initial platinum-based treatment.1
Efficacy / OS, PFS, and ORR results for Cyramza were consistent between the ITT population and patients with fast progression when added to docetaxel1,2
ITT Population (N=1253)2

43% of patients survived 1 year or longer with CYRAMZA plus docetaxel versus 38% with placebo plus docetaxel.
Major Outcome Measure - OS: Median Months (95% CI)2
- CYRAMZA + docetaxel (n=628): 10.5 months (95% CI: 9.5, 11.2)2
- Placebo + docetaxel (n=625): 9.1 months (95% CI: 8.4, 10.0)2
- HR=0.86 (95% CI: 0.75, 0.98; P=0.024)2
- 1.4-month survival difference2
- The percentage of deaths at the time of analysis was 68% (428 patients) and 73% (456 patients) in the CYRAMZA + docetaxel and placebo + docetaxel arms, respectively.
SELECT IMPORTANT SAFETY INFORMATION
Hemorrhage
- CYRAMZA increased the risk of hemorrhage and gastrointestinal hemorrhage, including Grade ≥3 hemorrhagic events. In 2137 patients with various cancers treated with CYRAMZA, the incidence of all Grade hemorrhage ranged from 13-55%. Grade 3-5 hemorrhage incidence ranged from 2-5%.
- Patients with NSCLC receiving therapeutic anticoagulation or with evidence of major airway invasion by cancer were excluded from REVEL. In addition, patients with NSCLC with a recent history of gross hemoptysis, those receiving chronic therapy with NSAIDs or other anti-platelet therapy other than once daily aspirin or with radiographic evidence of major airway or blood vessel invasion or intratumor cavitation were excluded from REVEL and RELAY; therefore the risk of pulmonary hemorrhage in these groups of patients is unknown.
- Permanently discontinue CYRAMZA in patients who experience severe (Grade 3 or 4) bleeding.
OS, PFS, and ORR results for CYRAMZA were consistent between the ITT population and patients with rapidly progressing disease† when added to docetaxel1,2
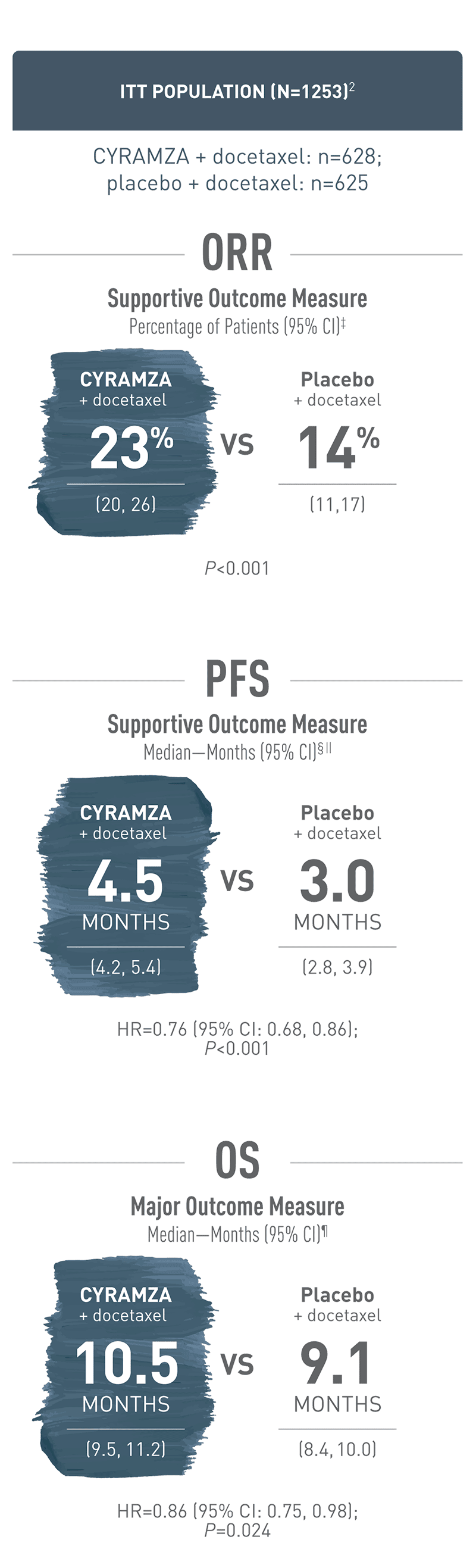
Overall response rate (ORR) was a supportive outcome measure of the REVEL trial. With CYRAMZA plus docetaxel in the intent-to-treat (ITT) population (n equals 628), ORR was 23 percent of patients with a 95 percent confidence interval of 20 to 26 percent. With placebo plus docetaxel in the ITT population (n equals 625), ORR was 14 percent of patients with a 95 percent confidence interval of 11 to 17 percent. The P value was less than 0.001. ORR was defined as complete response plus partial response. ORR did not include stable disease. Disease progression and tumor response were assessed by investigators in accordance with Response Evaluation Criteria in Solid Tumors (RECIST) 1.1.
Progression-free survival (PFS) was a supportive outcome measure of the REVEL trial. With CYRAMZA plus docetaxel in the intent-to-treat (ITT) population (n equals 628), median PFS was 4.5 months with a 95 percent confidence interval of 4.2 to 5.4 months. With placebo plus docetaxel in the ITT population (n equals 625), median PFS was 3.0 months with a 95 percent confidence interval of 2.8 to 3.9 months. The hazard ratio was 0.76 with a 95 percent confidence interval of 0.68 to 0.86 and a P value of less than 0.001. The percentage of events at the time of analysis was 89 percent (558 patients) in the CYRAMZA plus docetaxel treatment arm and 93 percent (583 patients) in the placebo plus docetaxel treatment arm. In CYRAMZA-treated patients, 126 of 558 events were deaths. In placebo-treated patients, 109 of 583 events were deaths.
Overall survival (OS) was the major outcome measure of the REVEL trial. With CYRAMZA plus docetaxel in the intent-to-treat (ITT) population (n equals 628), median OS was 10.5 months with a 95 percent confidence interval of 9.5 to 11.2 months. With placebo plus docetaxel in the ITT population (n equals 625), median OS was 9.1 months with a 95 percent confidence interval of 8.4 to 10.0 months. The hazard ratio was 0.86 with a 95 percent confidence interval of 0.75 to 0.98 and a P value of 0.024. The percentage of deaths at the time of the analysis was 68 percent (428 patients) in the CYRAMZA plus docetaxel treatment arm and 73 percent (456 patients) in the placebo plus docetaxel treatment arm of the trial.
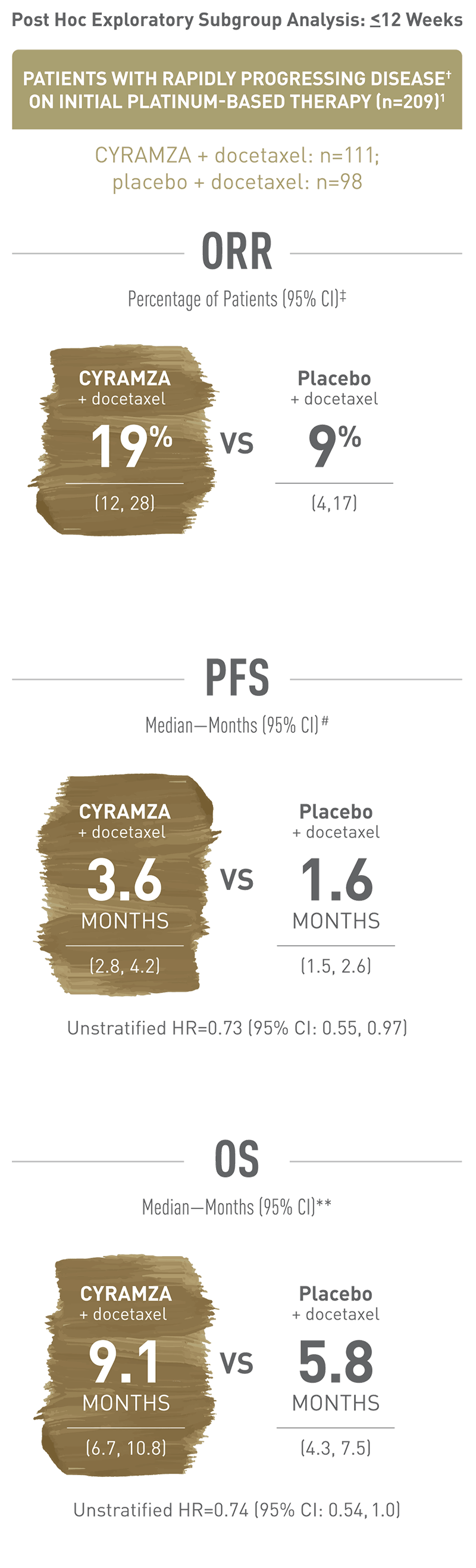
Rapidly progressing disease is defined as time to progression within 12 weeks after starting initial platinum-based treatment. This is a post hoc exploratory subgroup analysis of patients enrolled in the REVEL trial with rapidly progressing disease. With CYRAMZA plus docetaxel in patients with rapidly progressing disease (n equals 111), ORR was 19 percent of patients with a 95 percent confidence interval of 12 to 28 percent. With placebo plus docetaxel in patients with rapidly progressing disease (n equals 98), ORR was 9 percent of patients with a 95 percent confidence interval of 4 to 17 percent. ORR was defined as complete response plus partial response. ORR did not include stable disease. Disease progression and tumor response were assessed by investigators in accordance with Response Evaluation Criteria in Solid Tumors (RECIST) 1.1.
Rapidly progressing disease is defined as time to progression within 12 weeks after starting initial platinum-based treatment. This is a post hoc exploratory subgroup analysis of patients enrolled in the REVEL trial with rapidly progressing disease. With CYRAMZA plus docetaxel in patients with rapidly progressing disease (n equals 111), median PFS was 3.6 months with a 95 percent confidence interval of 2.8 to 4.2 months. With placebo plus docetaxel in patients with rapidly progressing disease (n equals 98), median PFS was 1.6 months with a 95 percent confidence interval of 1.5 to 2.6 months. The unstratified hazard ratio was 0.73 with a 95 percent confidence interval of 0.55 to 0.97. The percentage of events at the time of analysis was 91 percent (101 patients) in the CYRAMZA plus docetaxel treatment arm and 92.9 percent (91 patients) in the placebo plus docetaxel treatment arm.
Rapidly progressing disease is defined as time to progression within 12 weeks after starting initial platinum-based treatment. This is a post hoc exploratory subgroup analysis of patients enrolled in the REVEL trial with progressing disease. With CYRAMZA plus docetaxel in patients with rapidly progressing disease (n equals 111), median OS was 9.1 months with a 95 percent confidence interval of 6.7 to 10.8 months. With placebo plus docetaxel in patients with rapidly progressing disease (n equals 98), median OS was 5.8 months with a 95 percent confidence interval of 4.3 to 7.5 months. The unstratified hazard ratio was 0.74 with a 95 percent confidence interval of 0.54 to 1.0. The percentage of deaths at the time of the analysis was 75.7 percent (84 patients) in the CYRAMZA plus docetaxel treatment arm and 80.6 percent (79 patients) in the placebo plus docetaxel treatment arm of the trial.
† Rapidly progressing disease is defined as time to progression within 12 weeks after starting initial platinum-based treatment.1
‡ ORR=complete response plus partial response. ORR does not include stable disease. Disease progression and tumor response were assessed by investigators in accordance with RECIST 1.1.5
§ The percentage of events at the time of analysis was 89% (558 patients) and 93% (583 patients) in the CYRAMZA + docetaxel and placebo + docetaxel arms, respectively.2
|| 126 of 558 events in CYRAMZA-treated patients and 109 of 583 events in placebo-treated patients were deaths.2
¶ The percentage of deaths at the time of analysis was 68% (428 patients) and 73% (456 patients) in the CYRAMZA + docetaxel and placebo + docetaxel arms, respectively.2
** The percentage of deaths at the time of analysis in the CYRAMZA + docetaxel arm was 75.7% (84 patients) and 80.6% (79 patients) in the placebo + docetaxel arm.1,6
# The percentage of events at the time of analysis in the CYRAMZA + docetaxel arm was 91% (101 patients) and 92.9% (91 patients) in the placebo + docetaxel arm.1,6
REVEL Exploratory Analyses1,5,7
The REVEL trial was not adequately powered or error-controlled for subgroup analysis. Treatment differences observed in these subgroups cannot be regarded as statistically significant. The analysis described here were post hoc and exploratory
REVEL Trial Design (N=1253)
The phase III REVEL trial evaluated the efficacy and safety of CYRAMZA plus docetaxel vs placebo plus docetaxel in patients with locally advanced or metastatic NSCLC with disease progression on or after platinum-based chemotherapy. Major efficacy outcome measure was overall survival. Supportive efficacy outcome measures were progression-free survival and overall response rate. All patients were required to have Eastern Cooperative Oncology Group performance status 0 or 1. Patients were randomized 1:1 to receive either CYRAMZA 10 mg/kg (n=628) or placebo (n=625), in combination with docetaxel†† at 75 mg/m2 every 21 days.
†† 24 patients of East Asian sites received a starting dose of docetaxel at 60 mg/m2 every 3 weeks.
SELECT IMPORTANT SAFETY INFORMATION
Gastrointestinal Perforations
- CYRAMZA can increase the risk of gastrointestinal perforation, a potentially fatal event. In 2137 patients with various cancers treated with CYRAMZA, the incidence of all Grade and Grade 3-5 gastrointestinal perforations ranged from <1-2%.
- Permanently discontinue CYRAMZA in patients who experience a gastrointestinal perforation.
Watch Video: REVEL trial efficacy in ITT population
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Recommend ramucirumab (CYRAMZA) in combination with docetaxel (category 2A) as an option for metastatic NSCLC8
NCCN Guidelines® for mNSCLC recommend ramucirumab (CYRAMZA) in combination with docetaxel as a subsequent treatment option (category 2A) for metastatic NSCLC‡‡. Category 2A: Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.8
‡‡ For patients with metastatic NSCLC who are not candidates for targeted therapy or immunotherapy. For detailed recommendations, see the NCCN Guidelines for NSCLC.
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.

CI=confidence interval; ECOG=Eastern Cooperative Oncology Group; HR=hazard ratio; ITT=intent-to-treat; mNSCLC=metastatic non-small cell lung cancer; ORR=overall response rate; OS=overall survival; PFS=progression-free survival; PS=performance status; RECIST=Response Evaluation Criteria in Solid Tumors.
References
- Reck M, Shepherd F, Pérol M, et al. Effects of second-line ramucirumab after rapid time to progression on first-line therapy: subgroup analysis of REVEL in advanced non-small cell lung cancer. Oral presentation presented at: IASLC 18th World Conference on Lung Cancer; October 15-18, 2017; Yokohama, Japan.
- CYRAMZA (ramucirumab) package insert. Indianapolis, IN: Eli Lilly and Company; 2021.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
- Data on File, Lilly USA, LLC, DOF-RB-US-0049.
- Garon EB, Ciuleanu T-E, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665-673.
- Data on File, Lilly USA, LLC, DOF-RB-US-0045.
- Data on File, Lilly USA, LLC, DOF-RB-US-0006.
- Referenced with permission from The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer V.6.2020. © National Comprehensive Cancer Network, Inc. 2020. All rights reserved. Accessed June 19, 2020. To view the most recent and complete version of the guidelines, go online to https://www.nccn.org .